This section was published as:
Geissmann, T., 2000: Gibbon songs and human music from an evolutionary perspective.
In: The origins of music. Wallin, N.; Merker, B. & Brown, S. (eds.), pp. 103-123,
Cambridge, Massachusetts: MIT Press. ISBN 0-262-23206-5
A printable pdf-file of this paper can be downloaded here.
![]()
Gibbons (Hylobates spp.) produce loud and long song bouts that are mostly exhibited by mated pairs. Typically, mates combine their partly sex-specific reper toire in relatively rigid, precisely timed, and complex vocal interactions to produce well-patterned duets. A cross-species comparison reveals that singing behavior evolved several times independently in the order of primates. Most likely, loud calls were the substrate from which singing evolved in each line. Structural and behavioral similarities suggest that, of all vocalizations produced by nonhuman primates, loud calls of Old World monkeys and apes are the most likely candidates for models of a precursor of human singing and, thus, human music.
"Sad
the calls of the gibbons at the three gorges of Pa-tung;
After three calls in the night, tears wet the [traveller's] dress."
(Chinese song, 4th century, cited in Van Gulik, 1967, p. 46).
Of the gibbons or lesser apes, Owen (1868) wrote:
"... they alone, of brute Mammals, may be said to sing." Although a few
other mammals are known to produce songlike vocalizations, gibbons are among the
few mammals whose vocalizations elicit an emotional response from human listeners,
as documented in the epigraph.
The interesting questions, when comparing gibbon and human singing, are: do similarities
between gibbon and human singing help us to reconstruct the evolution of human music
(especially singing)? and are these similarities pure coincidence, analogous features
developed through convergent evolution under similar selective pressures, or the
result of evolution from common ancestral characteristics? To my knowledge, these
questions have never been seriously assessed.
Gibbons and Their Songs |
The gibbons or lesser apes form a highly specialized and homogenous group of primates.
Twelve gibbon species are currently recognized (Geissmann 1994, 1995) and are usually
combined in the family Hylobatidae within the Hominoidea, the group of primates that
includes apes and humans (figure 1).
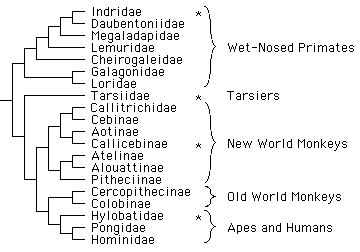
Figure 1
Phylogenetic tree of extant primate families and some subfamilies (phylogeny adapted from Purvis 1995; nomenclature after Groves 1993). Stars indicate singing and duet singing behavior, which is known of only four primate genera (Indri, Tarsius, Callicebus, Hylobates) representing four only distantly related species groups.
Gibbons are arboreal apes living in the tropical rain forests of south-east Asia.
Their specializations include, among others, a type of locomotion called brachiation.
Thus they are able not only to walk an branches but to locomote swiftly and economically
below branches, making them more efficient foragers in the thin-branch niche of trees
than other mammals of comparable body weight. Along with their locomotor specialization
are a large number of correlated anatomical adaptations, among which the elongation
of their arms and hands is most easily noticed (figure 2).
Gibbons have a monogamous social structure. Monogamy is quite unusual in mammals
and has been suggested to be a social characteristic of only approximately 3% of
species, in marked contrast to approximately 90% in bird species (Kleiman 1977).
As in most other monogamous species, gibbon groups usually consist of one adult pair
and one to three dependent offspring. These groups live in exclusive territories
that they actively defend. The most interesting specialization in gibbons, especially
with regard to the topic of this book, are their loud morning vocalizations, commonly
known as songs.
Figure 2
Singing male white-handed gibbon (Hylobates lar, Zoo Rapperswil).
For the purposes of this chapter, a song is what fulfills the criteria set forth
by Thorpe (1961:15): "What is usually understood by the term song is a series
of notes, generally of more than one type, uttered in succession and so related as
to form a recognizable sequence or pattern in time," or a succession of phrases
with nonrandom succession probability (Strophenfolgen mit nicht-zufälliger
Folgewahrscheinlichkeit, Tembrock 1977:33).
Gibbons produce loud and long song bouts. Depending on species and context, the bouts
have an average duration of ten to thirty minutes, but I also recorded an uninterrupted
song bout of a male Hylobates lar with a duration of eighty-six minutes. Songs
are preferentially uttered in the early morning hours, with species-specific preferences
for specific hours before, around or after dawn.
The songs are stereotyped and species-specific (Marshall and Marshall 1976, 1978;
Marler and Tenaza 1977; Haimoff 1984; Marshall and Sugardjito 1986; Geissmann 1993,
1995). Species can easily be identified by their songs (figure 3), and vocal characteristics
have been used to assess systematic relationships among hylobatids and reconstruct
their phylogeny (Haimoff at al. 1982, 1984; Creel and Preuschoft 1984; Marshall,
Sugardjito, and Markaya 1984; Geissmann 1993).
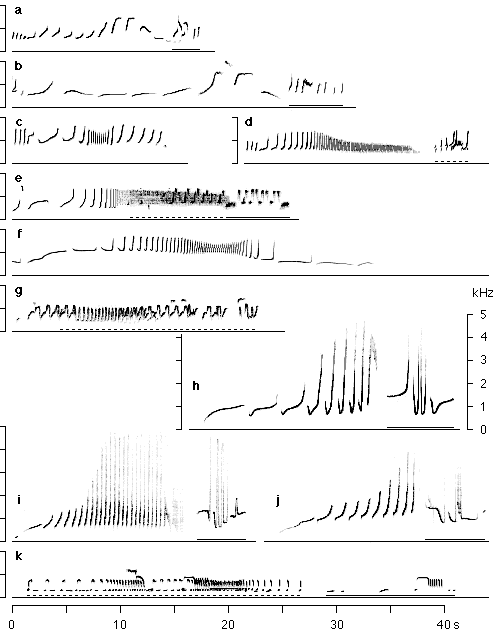
Figure 3
Sonagram of gibbon great call sequences. Sonagrams c and f are excerpts from female solo song bouts; all other sonagrams show duets. Male solo contributions to duets are underlined with a solid line, synchronous male and female vocalisations are underlined with a dashed line. a. Hylobates agilis (Asson Zoo); b. H. lar (Paignton Zoo); c. H. moloch (Munich Zoo), d. H. muelleri (Paignton Zoo); e. H. pileatus (Zürich Zoo); f. H. klossii (South Pagai, rec.: R.R. Tenaza); g. H. hoolock (Kunming Zoo); h. H. concolor (Xujiaba, Ailao Mountains); i. H. leucogenys (Paris, Ménagerie); j. H. l. gabriellae (Mulhouse Zoo); k. H. syndactylus (Metro Zoo, Miami).
Another specialization is the occurrence of duet singing in all gibbons with the
exception of H. klossii and H. moloch (Geissmann 1993). Duets are mostly
sung by mated pairs (figure 4). Typically, mates combine their repertoire in relatively
rigid, more or less precisely timed vocal interactions to produce well-patterned
duets.
Figure 4
A duetting pair of siamangs (Hylobates syndactylus, Munich Zoo).
Males of many gibbon species produce one or several distinct types of short phrases
that often become gradually more complex (e.g. in the number of notes, number of
distinct note types, degree of frequency modulation) as the song bout proceeds. At
more or less regular intervals, females insert long, female-specific phrases that
are commonly referred to as great calls. In most species, great calls consist of
a particularly rhythmic series of long notes uttered with increasing tempo and/or
increasing peak frequency. Males usually stop vocalising at the beginning of each
great call and provide a special reply phrase (coda) to the great call before resuming
their more common short phrases. In addition, one or both partners often exhibit
an acrobatic display at the climax of the great call, which may be accompanied by
piloerection and branch shaking (figure 5). The combination of the female great call
and the male coda is termed a great call sequence, and it may be repeated many times
during a single song bout.
Figure 5
Locomotor display of a male siamang (Hylobates syndactylus) during the duet song. Note piloerection (Munich Zoo).
Of course, this is a very simplified description of gibbon duetting. Most gibbon
species produce sequences other than great call sequences during a song bout. In
addition, females of most species contribute phrases other than great calls to the
duets, but because great calls (and great call sequences) are so loud and stereotyped,
most studies simply ignore the more variable portion of the female repertoire.
In the siamang (H. syndactylus) and possibly the hoolock (H. hoolock), duet interactions are considerably more complex - even within the great call sequence - than a simple great call-coda combination and include several different phrases and repeated vocal interactions between male and female (Geissmann, in press). According to Marshall and Sugardjito (1986:155) "the [siamang] duet is probably the most complicated opus sung by a land vertebrate other than man."
In contrast to what might be expected in primates and to what we know about song
development in many bird species, the species-specific characteristics in gibbons
are not learned, as demonstrated by studies on the vocal repertoire of a large number
of various hybrid gibbons (Geissmann 1984, 1993). A hybrid raised by its parents
in a zoo where no other gibbons are present receives only the male song of one parental
species and only the female song of the other parental species as potential templates
from which song learning would be possible.
For instance, a female hybrid between a male H. lar and a female H. muelleri never hears a great call other than that of H. muelleri. If great calls were learned, the hybrid should produce those of H. muelleri. If the parents are a male H. muelleri and a female H. lar, on the other hand, the hybrid will hear only great calls from H. lar and should end up producing those great calls. But neither of these options occurs (figure 6). Both types of hybrids produce the same, hybrid-specific types of great calls that combine elements of both parental species, although each hybrid has heard great calls of only one of the two species, and each had a different species as a potential template. This and similar results with male and female hybrids among various gibbon species clearly indicate that gibbons do not learn their repertoire from their parents.
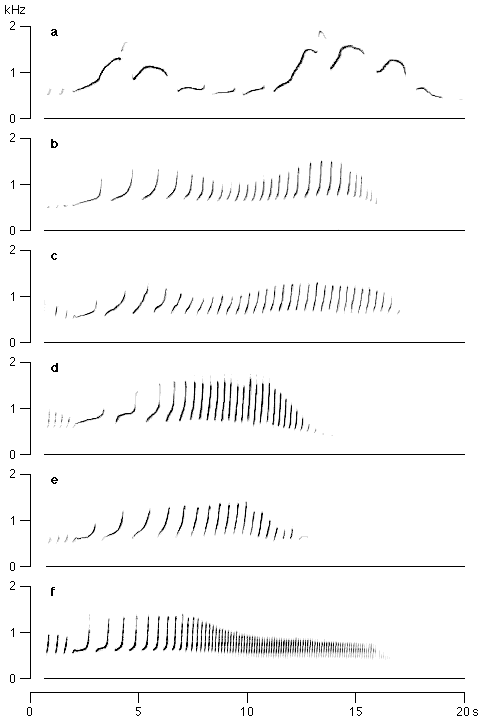
Figure 6
Sonagrams of female great calls of two gibbon species, Hylobates lar (a) and H. muelleri (f), and several unrelated first generation hybrids H. muelleri x H. lar (b-d) and H. lar x H. muelleri (e). a. H. lar (Al Maglio Zoo); b. Micky (Duisburg Zoo); c. no name (private owner, Mazé); d. Tina (Ravensden Farm, Rushden), e. no name (Micke Grove Zoo, rec.: R.R. Tenaza); f. H. muelleri (Paignton Zoo).
Clearly, song serves more than one function in birds and gibbons. Marshall and Marshall
(1976) proposed that different selection pressures act on male and female repertoires
in gibbon duets. Possibly, different parts of the same individual's duet contribution
may also differ in function (Goustard 1985).
Apparently, most songs are produced either without any recognisable external stimulus or in response to songs of neighboring groups. Only occasionally are they produced in response to alarming situations (I repeatedly observed Hainan crested gibbons directing great calls to me).
Functions most frequently suggested for duet songs include territorial advertisement and strengthening of pair bonds (Chivers 1976; Farabaugh 1982; Brockelman and Srikosamatara 1984; Mitani 1985a). The latter in particular is a matter of debate (Cowlishaw 1992) and "has not yet been demonstrated in any animal species that sings" (Haimoff 1983:iv). According to Brockelman (1984:286), "this function of duetting is poorly understood, for it is not clear how exactly duets would do this, or what kind of evidence would support the idea. In short, there is no explicit paradigm for analysing such communicative behavior."
Wickler (1980) first suggested a plausible mechanism by which duet songs could affect the cohesiveness of the pair bond. If duetting has to be learned at the beginning of each pair formation, this would reduce the probability of partner desertion, since learning investment would have to be provided anew with every new partner. To support this pair-bonding hypothesis, the following three conditions must be met: duet amelioration after pair formation has to be a necessary precondition to copulation; duets have to be pair-specific; and pair-specificity must be based on a mate-specific duetting-relationship of at least one mate. To test these predictions, changes in duet structure in two pairs of siamangs (H. syndactylus) during a forced partner exchange were examined (Geissmann, in preparation). The two newly formed pairs appear to be the first documented cases to fulfill the requirements underlying Wickler's (1980) pair-bonding hypothesis: the animals showed a stable song pattern with pair-specific traits. After the partner exchange, new pair-specific traits occurred, some of them apparently achieved through a directed effort of one or both individuals.
That study did not prove, however, that duetting in siamangs strengthens the pair bond, because evidence of a direct relationship between the pair bond strength and quality of duetting is lacking. If duetting is related to pair bonding, one would expect to find a relationship between its intensity and indicators of pair bond strength. To test this, daily frequency and duration of duetting and three generally accepted indicators of pair bond strength (mutual grooming, behavioral synchronization and interindividual distance between mates) were recorded in 10 siamang groups observed in various zoos (Geissmann and Orgeldinger 1998, in preparation). This revealed that duetting activity was positively correlated with grooming activity and behavioral synchronization, and negatively correlated with interindividual distance between mates. These results suggest that production of coordinated duets by siamang pairs is indeed related to pair bonding.
As mentioned, considerable differences exist among gibbon species in the complexity of song structure and interaction rules, ranging from species that produce solo songs only (e.g., H. klossii), to those with a relatively simple duet structure (e.g., H. leucogenys), to the siamang with its highly complex vocal interactions. These differences indicate that song bouts also differ in their functions or in the importance of these functions, and interpretations on one species may not necessarily apply to all species. If the complex duet song of the siamang serves, among other functions, to strengthen the pair bond, this function may not necessarily apply to gibbons of the lar group or the concolor group, whose simpler duet structure may not require practising among newly mated animals. Strengthening of the pair bond may indeed be a highly specialized function of the siamang duet song. The loudness of this song suggests, however, that other functions are also involved. These are most probably related to pair territorial advertisement, bond advertisement and possibly mate attraction (Geissmann, in preparation).
In birds, experimental evidence supports the notion
that songs function as a courtship display in at least some species. In whales, only
males appear to sing. Here, the song may function less as a courtship display, but
rather play a role in male-male competition (K. Payne, personal communication). In
all singing primates (Indri, Tarsius, Callicebus, Hylobates:
see below), on the other hand, females contribute to singing often as much as the
males. Experimental data failed to support the hypothesis that gibbon songs may have
a mate-attracting function (Mitani 1988). It has repeatedly been observed, on the
other hand, that subadult males in wild H. agilis, H. lar, H. klossii
and H. syndactylus tend to sing more often, for longer durations, or earlier
in the morning than mated males (Aldrich-Blake and Chivers 1973; Ellefson 1974; Tenaza
1976; Gittins 1978; Tilson 1981; Raemaekers and Raemaekers 1984; Raemaekers, Raemaekers,
and Haimoff 1984; personal observation). In another siamang group in Sumatra, the
subadult male was twice observed producing solo songs within the territory of his
family group before his dispersal (Palombit 1992:319).
Phylogenetic Comparisons |
In contrast to birds, singing behavior is rare in mammals and, among primates, is
known only for members of the following four genera - Indri, Tarsius,
Callicebus, and Hylobates (Robinson 1979, 1981; MacKinnon and MacKinnon
1980; Haimoff 1986; Niemitz et al. 1991; Geissmann 1993; Thalmann et al. 1993; Müller
1994, 1995; Nietsch and Kopp 1998). These singing primates comprise about twenty-six
species (depending on the currently accepted taxonomy), amounting to about 11% of
primate species or 6% of primate genera.
In all singing primates, males and females both sing, and in most singing primates, duet singing occurs. It is interesting to note that all primate species that are known to sing are also thought to have a monogamous social structure. In birds, too, duet songs mainly occur in monogamous species. This suggests that the evolution of singing behavior in primates and of duet singing behavior in general are somehow related to the evolution of monogamy.
Since the four species groups of primates that exhibit singing (and duet singing) behavior are not closely related, it is likely that singing (and duet singing) evolved four times independently within the order of primates.
Long, loud, and complex song bouts have been described for all gibbon species. What
did ancestral gibbons sound like? It is probably safe to assume that vocal characteristics
shared by all modern gibbon species were also present in their last common ancestor.
Just what are these common characteristics? Gibbon songs consist of phrases which
are typically pure in tone and with energy concentrated in the fundamental frequency.
Depending on species, the fundamental frequency of song vocalizations ranges between
0.2 and 5 kHz. During the song bout, male song contributions exhibit some form of
gradual development from initially simpler phrases to increasingly complex phrases.
Females contribute a stereotyped great call phrase and exhibit a ritualized locomotor
display at the climax of the great call. In many species, the male contributes a
vocal coda to the female's great call and may also participate in the display.
A comparative phylogenetic analysis of gibbon songs, taking into consideration comparative characteristics of loud calls of other Old World monkeys and apes, came to the following conclusions concerning the evolution of gibbon songs (Geissmann 1993). The recent hylobatids represent a monophyletic group whose common ancestor produced duet songs, although not all recent species are known to duet. Duet songs of recent gibbon species are likely to have evolved according to the song-splitting theory (a term coined by Wickler and Seibt 1982). Accordingly, the duets probably evolved from a song that was common to both sexes and only later became separated into male-specific and female-specific parts. In addition, a process tentatively called duet-splitting is suggested to have led secondarily from a duetting species to non-duetting species such as H. klossii and H. moloch, in that the contributions of the partners split into temporally segregated solo songs.
Great calls of all gibbon species are, indeed, a homologous song phrase. The acceleration of the rate of note emission during the great call (and possibly the subsequent slow-down in rhythm near the end of the call) are probably the ancestral condition. The ancestor of modern gibbons probably produced great calls with a relatively moderate acceleration similar to that of H. moloch. The use of biphasic notes (alternate production of exhalation and inhalation sounds) probably represents a primitive characteristic for both male and female gibbon vocalizations. Biphasic notes are dominant in the female great calls of H. hoolock and H. syndactylus, and they also occur rarely in H. agilis, H. lar and H. moloch. These types of notes are also dominant in the male song of H. hoolock, H. agilis, and H. pileatus, and occur occasionally in H. lar and H. moloch as well (figure 7).
Great apes and humans are usually recognized as being the phylogenetic sister group
to the gibbons. Among members of this group, some vocalizations can be discerned
that at least in part resemble elements of the gibbon song (i.e., the great call)
in their presumed functions and to a lesser degree in structure. These vocalizations
are thought to be used primarily in interindividual or intergroup spacing.
In orangutans (Pongo pygmaeus), long calls are given by males only, and are often accompanied by piloerection and branch-shaking displays. Calls last up to one minute in Sumatra and up to three minutes in Borneo. Their frequency is concentrated below .7kHz in Sumatra and below 1.3kHz in Borneo. Long calls begin with a short series of low-frequency, low-intensity bubbling notes that build up to a long series of evenly spaced, high-intensity moans or roars, then tail off gradually in another series of bubbling notes. The number of notes is rarely more than twenty-five in Sumatra, but sometimes up to fifty in Borneo. Bubbling inhalation notes occur in the inhalatory pauses between the roars (Rijksen 1978). Long calls are mostly produced during the night in Sumatra, but during the daytime in Borneo with a peak between 9:00 and 10:00 A.M. They are the only orangutan vocalization that can be heard over long distances and are hypothesized to mediate interindividual spacing among males (Brandes 1931; Hofer 1972; MacKinnon 1974; Rijksen 1978; Galdikas 1983; Mitani 1985b; Roché 1994).
In gorillas (Gorilla gorilla), hoot series are most frequently given by silverback males and may be terminated by chest beating, branch breaking, or runs through thick foliage. Hoot series last only a few seconds (Schaller 1963; Fossey 1972, 1983; Hess 1988; Roché 1994; Bouchain and Gautier 1995) with frequency concentrated between 1 and 1.8kHz. They typically consist of two to twenty, but exceptionally up to eighty-four, hoots that may become slurred at the end, blending into a growling sound. Hoots are often presented in accelerated series, with the individual sounds resembling a bubbling trill at the climax. Hoot-series often begin softly and with low frequency, but intensity and frequency build up during a call. Inhalation notes were not reliably recognized in the recordings and sonagrams available during this study. Hoot series are fairly loud and "may travel for roughly a mile" (Fossey 1983). This call has been hypothesized to be used primarily in long-range intergroup communication.
In common chimpanzees (Pan troglodytes), a distinctive loud call known as the pant-hoot is uttered by both sexes and all ages, but most often by males (Marler 1969; Marler and Hobbett 1975; Marler and Tenaza 1977; Goodall 1986, Mitani et al. 1992; Roché 1994). Pant-hoots last from two to twenty-three seconds and their fundamental frequency ranges from .2 to 1kHz. Pant-hoots have four distinct phases. Calls may begin with a brief introduction consisting of a series of unmodulated tonal elements of low frequency. A progressively louder build-up follows, containing elements that are typically shorter than those in the introduction and produced both on inhalation and exhalation (figure 7f). Some further acceleration in rhythm may occur during this phase. The third phase, the climax, is characterized by one or several long, frequency-modulated elements resembling a scream in acoustic properties. This section is frequently present during pant-hooting of male chimpanzees and typically absent in females. Frequency reaches its peak in this phase. It is often accompanied by a vigorous charging display, which may include erection of hair, running along the ground, dragging or flailing branches, throwing rocks or other loose material, slapping the ground with hands, stomping with feet, hitting or stamping at a tree (drumming display), seizing branches and swaying them vigorously from side to side, or showing exaggerated leaps or brachiation in a tree (Goodall 1986). Pant-hoots conclude with a let-down portion, which includes unmodulated tonal elements of low frequency, similar to those of the build-up.
Pant-hooting is given in several contexts, including in response to other pant-hooting individuals, after rejoining other community members, in response to strange conspecifics, on arriving at a particularly rich food source, during agonistic displays, on capturing prey items, and during the night. It can be heard over long distances and its functions have been hypothesized to include long-range announcement of an individual's presence and sex, hence mediating interindividual spacing among some individuals and groups, and reunion of others. In bonobos (P. paniscus), apparently homologous vocalizations are known under the term hooting complex and occur in similar contexts as pant-hooting of common chimpanzees (de Waal 1988).
Characteristics of these great ape calls resembling at least some gibbon songs (especially the female great calls) include loudness, a hypothetical function in long-distance interindividual or intergroup communication (all species), acceleration of note rhythm (common in chimpanzees and gorillas, apparently absent in orangutans), a final slow-down in rhythm (chimpanzees), higher intensity in the central section of the call (apparently in all species of great apes, but variable in orangutans), biphasic notes consisting of alternating exhalation and inhalation (absent in gorillas), higher frequency in the central section of the call, pure tone of notes (most prominent in chimpanzees), and frequent accompaniment with piloerection and a locomotor display that may include leg kicking, stomping, branch shaking, vegetation slapping or throwing, jumping, running, chest beating, or ground thumping.
Among members of the Old World monkeys, too, certain vocalizations can be discerned that resemble some elements of the gibbon song (great call) in function and to some degree in structure. In many species these characteristics are restricted to loudness and a hypothetical function in long-distance interindividual or intergroup communication (Vogel 1973; Horwich 1976; Tilson and Tenaza 1976; Waser 1977, 1982; Oates and Trocco 1983; Herzog and Hohmann 1984; Hohmann and Herzog 1985; Gautier 1988). Other characteristics mentioned above are frequently absent. In many species (Cercocebus spp., Lophocebus spp., Macaca silenus, Papio spp., Presbytis potenziani, P. thomasi, Simias concolor, Trachypithecus spp.) the occurrence of biphasic notes consisting of alternating exhalation and inhalation has been reported. In some species (Cercocebus galeritus, Macaca silenus, Trachypithecus johnii, Semnopithecus entellus) notes are remarkably pure in tone, and in some (Cercocebus galeritus, Trachypithecus johnii) they are produced with accelerating rhythm. In addition, these calls are often supplemented with a ritualized locomotor display (Vogel 1973; Horwich 1976; Tilson and Tenaza 1976; Tilson 1977; Byrne 1981; Waser 1982; Herzog and Hohmann 1984; Steenbeek and Assink 1998).
Among great apes, chimpanzee pant-hooting apparently shares most similarities with gibbon great calls. Among Old World monkeys, similarities with great calls are particularly prominent in the whooping display of the Nilgiri langur (Trachypithecus johnii) and some other Asian colobines. These similarities do not necessarily imply homology, but it is tempting to assume that loud calls with biphasic notes and an accelerated rate of note emission followed by a slowing down represent the ancestral condition of hominoids, and perhaps even of Old World monkeys.
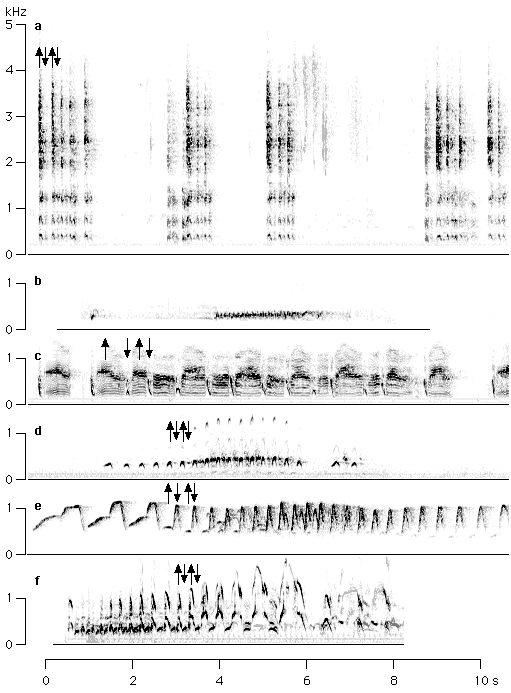
Figure 7
The occurrenc of biphasic notes in loud calls (or excerpts of loud calls) of Old World monkeys (a-d) and apes (e-f). Alternating exhalation and inhalation notes are indicated by upward and downward arrows, respectively. In (b), no arrows are used, because exhalation and inhalation notes are produced very rapidly in this example. a. Chlorocebus aethiops (two individuals, South Africa; Roché, 1994); b. Lophocebus albigena (adult male, Gabon, Bouchain and Gautier, 1995); c. Colobus satanas (Gabon; Roché, 1994), d. Trachypithecus johnii (India; rec.: G. Hohmann); e. Hylobates hoolock (adult female and juvenile male, Kunming Zoo); f. Pan troglodytes (Gambia; Roché, 1994).
Long, uninterrupted vocal bouts that correspond to the definition of songs are, however,
not known for any of these species. The sequential nature of female solo song bouts
and duet song bouts, as well as the gradual development of increasingly complex phrases
observed in male solo song bouts, appear to be synapomorphic characteristics of gibbons
not reported for other Old World monkeys and apes. It should also be noted that the
loud calls of most Old World monkeys and great apes described above are mainly male-specific
vocalizations or preferentially uttered by males, whereas their main structural similarities
to gibbon songs are concentrated on great calls, which are essentially female-specific.
The occurrence of female loud calls may to some degree be related to the monogamous
mating system of gibbons. In addition, the gap between male and female loud calls
is reduced to some extent by the observation that pant-hooting also occurs in female
chimpanzees (see above), whereas male gibbons of the concolor group, and occasionally
of other gibbon species as well, typically produce great call-like phrases before
reaching adulthood (personal observation). Moreover, loud calls of male Mentawai
langurs (Presbytis potenziani) directed toward adjacent groups may be supplemented
by a facultative coda of three to four loud, apparently pure tones produced by the
female, hence forming a simple duet (Tilson and Tenaza 1976).
Most primate species produce specific, at least
partly stereotyped loud calls in territorial or alarming contexts. It may be speculated
that the alternate use of inhalation and exhalation notes may be ancestral to an
even larger taxonomic group than just Old World monkeys and apes, maybe to all primates.
Although the available evidence is inconclusive, it should be noted that biphasic
vocalizations are apparently used in loud calls by some New World monkeys (Alouatta,
Callicebus) and wet-nosed primates (Propithecus, Avahi) (personal
observation).
A Link to Human Music? |
As pointed out above, singing behavior appears to have developed several times in primate evolution. Both the context in which singing occurs in nonhuman primates and the structure of some song contributions show similarities to territorial calls or alarm calls in nonsinging species. This suggests that singing in primates evolved each time from loud calls used in a territorial or alarm context. It makes sense to assume that the same applies to the evolution of human singing behavior, and that loud calls of early hominids may have been the substrate from which human singing and, ultimately, music evolved.
Most forms of music are tied to emotionality and
have a powerful effect on both the audience and the performer, compelling them to
shake body parts to the rhythm, beat the rhythm by clapping or stomping, or locomote
(dance) to the rhythm. Often, dancing appears to be inseparably linked with music
(Ewens 1995). The almost universal, almost hypnotic effect of music on most humans
suggests that this is an ancestral characteristic that may have a strong inherited
component. In addition, this behavior bears an obvious similarity to the ritualized
locomotor displays (drumming, stomping, branch shaking) associated with loud calls
of many Old World primates, providing additional support that music is derived from
loud calls.
It is tempting to assume that early hominid singing shared many characteristics with
loud calls of modern Old World monkeys and especially apes, such as loudness for
long distance communication, pure tonal quality of notes, use of stereotyped phrases,
use of biphasic notes, accelerando in note-rhythm and possibly a slow down near the
end of the phrase, a locomotor display, and a strong inherited component.
After the divergence between early humans and some forms of African apes from a common
ancestor, several characteristics of human music evolved that are not found in loud
calls of modern monkeys and apes. The most conspicuous of these are a steady rhythm
(pulse, beat), reduction of inherited stereotypy in favor of increased importance
of learning phrases and sequence rules, and the option to invent new signal patterns
(improvisation) and new conventions (exact repetition of improvised units) spontaneously.
Universals of human music are difficult to identify but probably include a steady,
accentuated beat (see Arom, Nettl, and Mâche, this volume). Although some primates
are able to produce short, monosyllabic calls for several seconds at a relatively
steady pulse (e.g. some galagos, Galagonidae; E. Zimmermann, personal communication)
and mouse lemurs (Cheirogaleidae, personal observation), nonhuman primates, unlike
humans, do not seem to be able to keep a steady pulse in their song vocalizations.
There is an interesting report on pulse-keeping behavior in a female white-handed
gibbon (H. lar). This zoo animal was observed to follow the beats of a metronome
with its short calls (Ziegler and Knobloch 1968) as long as the speed remained within
the limits of 60 to 122 (the authors probably referred to beats per minute). Outside
of these tolerance limits, the animal produced short notes at a rhythm of approximately
112. The gibbon's response was best at a metronome tempo of 60, and not when presented
with its own normal speed of 112. The relevance of this observation is difficult
to assess. The authors provided no sonagrams of the vocalizations, but the description
may refer to a form of contact call rather than a song vocalization.
What fitness advantage is there to add a steady beat to a song vocalization? The
beat may help larger social groups to participate in a song, to coordinate it. A
well-coordinated song may be a more effective display than a cacophony of voices,
and other social groups are less likely to attack or threaten well coordinated groups.
In addition, introduction of a steady beat may make it easier to assess a group's
cohesiveness and therefore its strength based on the group display.
The main message of this chapter is that loud calls in modern apes and music in modern
humans are derived from a common ancestral form of loud call. If this interpretation
is correct, early hominid music may also have served functions resembling those of
ape loud calls. Loud calls are believed to serve a variety of functions, including
territorial advertisement; intergroup intimidation and spacing; announcing the precise
locality of specific individuals, food sources, or danger; and strengthening intragroup
cohesion. The most widely distributed (albeit not universal) function, and probably
the most likely function of early hominid music, is to display and possibly reinforce
the unity of a social group toward other groups. In humans, this function is still
evident today whenever groups of people, be they united by political, religious,
age, or other factors, define themselves by their music. National hymns, military
music, battle songs of fans and cheerleaders encouraging their favorite sports teams,
or the strict musical preferences of youth gangs may serve as examples of this phenomenon,
whose origin may go back to the very beginning of human evolution.
Acknowledgments |
Aldrich-Blake, F. P. G. and Chivers, D. J. (1973). On the genesis of a group of siamang.
American Journal of Physical Anthropology 38, 631-636.
Bouchain, C. and Gautier, J.-P. (1995). Le Monde des singes / Primate World Vol.
2. Compact disk. Mens, France: Sitelle.
Brandes, R. (1931). Über den Kehlkopf des Orang-Utan in verschiedenen Altersstadien
mit besonderer Berücksichtigung der Kehlsackfrage. Morphologisches Jahrbuch
69, 1-61, + 3 plates.
Brockelman, W. Y. (1984). Social behaviour of gibbons: Introduction. In H. Preuschoft,
D. J. Chivers, W. Y. Brockelman and N. Creel (Eds.), The lesser apes. Evolutionary
and behavioural biology (pp. 285-290). Edinburgh: Edinburgh University Press.
Brockelman, W. Y. and Srikosamatara, S. (1984). Maintenance and evolution of social
structure in gibbons. In H. Preuschoft, D. J. Chivers, W. Y. Brockelman and N. Creel
(Eds.), The lesser apes. Evolutionary and behavioural biology (pp. 298-323). Edinburgh:
Edinburgh University Press.
Byrne, R. W. (1981). Uses of long-range calls during ranging by Guinea baboons. In
A. B. Chiarelli and R. S. Corruccini (Eds.), Primate behavior and sociobiology (pp.
104-109). Berlin and New York: Springer Verlag.
Chivers, D. J. (1976). Communication within and between family groups of siamang
(Symphalangus syndactylus). Behaviour 57, 116-135.
Cowlishaw, G. (1992). Song function in gibbons. Behaviour 121, 131-153.
Creel, N. and Preuschoft, H. (1984). Systematics of the lesser apes: A quantitative
taxonomic analysis of craniometric and other variables. In H. Preuschoft, D. J. Chivers,
W. Y. Brockelman and N. Creel (Eds.), The lesser apes. Evolutionary and behavioural
biology (pp. 562-613). Edinburgh: Edinburgh University Press.
Ellefson, J. O. (1974). A natural history of white-handed gibbons in the Malayan
Peninsula. In D. M. Rumbaugh (Ed.), Gibbon and siamang, vol. 3 (pp. 1-136). Basel
and New York: Karger.
Ewens, G. (1995). Die Klänge Afrikas. Zeitgenössische Musik von Kairo bis
Kapstadt (T. Brückner, Trans.). München: Marino Verlag. (Original work
published 1991).
Farabaugh, S. M. (1982). The ecological and social significance of duetting. In D.
E. Kroodsma, E. H. Miller and H. Ouellet (Eds.), Acoustic communication in birds
(pp. 85-124). New York and London: Academic Press.
Fossey, D. (1972). Vocalizations of the mountain gorilla (Gorilla gorilla beringei).
Animal Behavior 20, 36-53.
Fossey, D. (1983). Gorillas in the mist. Boston: Houghton Mifflin Company.
Galdikas, B. M. F. (1983). The orangutan long call and snag crashing at Tanjung Puting
Reserve. Primates 24, 371-384.
Gautier, J.-P. (1988). Interspecific affinities among guenons as deduced from vocalizations.
In A. Gautier-Hion, F. Bourlière, J.-P. Gautier and J. Kingdon (Eds.), A primate
radiation: Evolutionary biology of the African guenons (pp. 194-226). Cambridge:
Cambridge University Press.
Geissmann, T. (1984). Inheritance of song parameters in the gibbon song, analyzed
in 2 hybrid gibbons (Hylobates pileatus x H. lar). Folia Primatologica
42, 216-235.
Geissmann, T. (1993). Evolution of communication in gibbons (Hylobatidae). Ph.D.
thesis, Anthropological Institute, Philosoph. Faculty II, Zürich University.
Geissmann, T. (1994). Systematik der Gibbons. Zeitschrift des Kölner Zoo 37,
65-77.
Geissmann, T. (1995). Gibbon systematics and species identification. International
Zoo News 42, 467-501.
Geissmann, T. (In press). Duet songs of the siamang, Hylobates syndactylus:
I. Structure and organisation. Primate Report.
Geissmann, T. (In preparation). Duet songs of the siamang, Hylobates syndactylus:
II. Testing the pair-bonding hypothesis during a partner exchange.
Geissmann, T. and Orgeldinger, M. (1998). Duet or divorce! [abstr]. Folia Primatologica
69, 283.
Geissmann, T. and Orgeldinger, M. (In preparation). Duet or divorce: The relationship
between duet singing and the pair bond.
Gittins, S. P. (1978). Hark! The beautiful song of the gibbon. New Scientist 80,
832-834.
Goodall, J. (1986). The chimpanzees of Gombe. Patterns of behavior. Cambridge, Massachusetts,
and London: The Bellknap Press of University Press.
Goustard, M. (1985). Structure acoustique et fonctions des vocalisations territoriales,
chez le Gibbon à mains blanches (Hylobates lar), observé dans
son habitat naturel, en Thaïlande. Annales des Sciences Naturelles, Zoologie,
Paris, 13e série 7 265-279.
Groves, C. P. (1993). Order Primates. In D. E. Wilson and D. M. Reader (Eds.), Mammal
species of the world. A taxonomic and geographic reference, 2nd. ed. (pp. 243-277).
Washington, D.C.: Smithsonian Institution Press.
Haimoff, E. H. (1983). Gibbon songs: An acoustical, organizational, and behavioural
analysis. PhD. diss., University of Cambridge.
Haimoff, E. H. (1984). Acoustic and organizational features of gibbon songs. In H.
Preuschoft, D. J. Chivers, W. Y. Brockelman and N. Creel (Eds.), The lesser apes.
Evolutionary and behavioural biology (pp. 333-353). Edinburgh: Edinburgh University
Press.
Haimoff, E. H. (1986). Convergence in the duetting of monogamous Old World primates.
Journal of Human Evolution 15, 51-59.
Haimoff, E. H., Chivers, D. J., Gittins, S. P. and Whitten, A. J. (1982). A phylogeny
of gibbons based on morphological and behavioural characters. Folia Primatologica
39, 213-237.
Haimoff, E. H., Gittins, S. P., Whitten, A. J. and Chivers, D. J. (1984). A phylogeny
and classification of gibbons based on morphology and ethology. In H. Preuschoft,
D. J. Chivers, W. Y. Brockelman and N. Creel (Eds.), The lesser apes. Evolutionary
and behavioural biology (pp. 614-632). Edinburgh: Edinburgh University Press.
Herzog, M. O. and Hohmann, G. M. (1984). Male loud calls in Macaca silenus
and Presbytis johnii. A comparison. Folia Primatologica 43, 189-197.
Hess, J. (1988). Berggorillalaute. Akustische Erinnerungen an die Berggorillas der
Familie 5 (audio cassette). Fuchsmattstr. 27, CH - 4107 Ettingen: HM Produktion.
Hofer, H. (1972). Über den Gesang des Orang-Utan. Der Zoologische Garten (N.F.)
41, 299-302.
Hohmann, G. M. and Herzog, M. O. (1984). Vocal communication in lion-taled macaques
(Macaca silenus). Folia Primatologica 4, 148-178.
Horwich, R. H. (1976). The whooping display in Nilgiri langurs: An example of daily
fluctuations superimposed on a general trend. Primates 17, 419-431.
Kleiman, D. G. (1977). Monogamy in mammals. Quarterly Review of Biology 52, 39-69.
MacKinnon, J. (1974). The behaviour and ecology of wild orang-utan (Pongo pygmaeus).
Animal Behavior 22, 3-74.
MacKinnon, J. and MacKinnon, K. (1980). The behavior of wild spectral tarsiers. International
Journal of Primatology 1, 361-379.
Marler, P. (1969). Vocalizations of wild chimpanzees. An introduction. In C. R. Carpenter
(Ed.), Proceedings of the Second International Congress of Primatology, Atlanta,
GA 1968. vol. 1: Behavior (pp. 94-100). Basel and New York: Karger.
Marler, P. and Hobbett, L. (1975). Individuality in a long-range vocalization of
wild chimpanzees. Zeitschrift für Tierpsychologie 38, 97-109.
Marler, P. and Tenaza, R. (1977). Signaling behavior of apes with special reference
to vocalization. In T. A. Sebeok (Ed.), How animals communicate (pp. 965-1033). Bloomington
and London: Indiana University Press.
Marshall, J. T. and Marshall, E. R. (1976). Gibbons and their territorial songs.
Science 193, 235-237.
Marshall, J. T. and Marshall, E. R. (1978). The gibbons (phonograph disc). J.W. Hardy
and C.K. Hardy (Eds.) 1615 NW 14th Ave., Gainesville, FL 32605, USA: ARA-Records.
Marshall, J. T. and Sugardjito, J. (1986). Gibbon systematics. In D. R. Swindler
and J. Erwin (Eds.), Comparative primate biology, vol. 1: Systematics, evolution,
and anatomy (pp. 137-185). New York: Alan R. Liss.
Marshall, J. T., Sugardjito, J. and Markaya, M. (1984). Gibbons of the lar group:
Relationships based on voice. In H. Preuschoft, D. J. Chivers, W. Y. Brockelman and
N. Creel (Eds.), The lesser apes. Evolutionary and behavioural biology (pp. 533-541).
Edinburgh: Edinburgh University Press.
Mitani, J. C. (1985a). Gibbon song duets and intergroup spacing. Behaviour 92, 59-96.
Mitani, J. C. (1985b). Sexual selection and adult male orangutan long calls. Animal
Behavior 33, 272-283.
Mitani, J. C. (1988). Male gibbon (Hylobates agilis) singing behavior: Natural
history, song variations and function. Ethology 79, 177-194.
Mitani, J. C., Hasegawa, T., Gros-Louis, J., Marler, P. and Byrne, R. (1992). Dialects
in wild chimpanzees? American Journal of Primatology 27, 233-243.
Müller, A. (1994). Duettieren beim Springaffen (Callicebus cupreus).
Diploma thesis, Anthropological Institute, Zürich University.
Müller, A. (1995). Duetting in the titi monkey Callicebus cupreus. Neotropical
Primates 3(1), 18-19.
Niemitz, C., Nietsch, A., Warter, S. and Rumpler, Y. (1991). Tarsius dianae:
A new primate species from Central Sulawesi (Indonesia). Folia Primatologica 56,
105-116.
Nietsch, A. and Kopp, M.-L. (1998). Role of vocalization in species differentiation
of Sulawesi Tarsiers. Folia Primatologica 69 (Supplement 1), 371-378.
Oates, J. F. and Trocco, T. F. (1983). Taxonomy and phylogeny of black-and-white
colobus monkeys. Inferences from an analysis of loud call variation. Folia Primatologica
40, 83-113.
Owen, R. (1868). On the anatomy of vertebrates, vol. 3: Mammals. London: Longmans,
Green and Co.
Palombit, R. A. (1992). Pair bonds and monogamy in wild siamang (Hylobates syndactylus)
and white-handed gibbon (Hylobates lar) in northern Sumatra. Ph.D. thesis, University
of California, Davis.
Purvis, A. (1995). A composite estimate of primate phylogeny. Philosophical Transactions
of the Royal Society of London B 348, 405-421.
Raemaekers, J. J. and Raemaekers, P. M. (1984). Vocal interactions between two male
gibbons, Hylobates lar. Natural History Bulletin of the Siam Society 32, 95-106.
Raemaekers, J. J., Raemaekers, P. M. and Haimoff, E. H. (1984). Loud calls of the
gibbon (Hylobates lar): Repertoire, organization and context. Behaviour 91,
146-189.
Rijksen, H. D. (1978). A field study on Sumatran orang utans (Pongo pygmaeus abelii
Lesson 1827). Ecology, behaviour and conservation. Mededelingen Landbouwhogeschool,
Wageningen 78 (2) Wageningen: Veenman and Zonen.
Robinson, J. G. (1979). An analysis of the organization of vocal communication in
the titi monkey, Callicebus moloch. Zeitschrift für Tierpsychologie 49,
381-405.
Robinson, J. G. (1981). Vocal regulation of inter- and intragroup spacing during
boundary encounters in the titi monkey, Callicebus moloch. Primates 22, 161-172.
Roché, J. C. (1994). Le Monde des singes / Primate World Vol. 1. Compact disk.
Mens, France: Sitelle.
Schaller, G. B. (1963). The mountain gorilla. Ecology and behavior. Chicago: University
of Chicago Press.
Steenbeek, R. and Assink, P. (1998). Individual differences in long-distance calls
of male wild Thomas langurs (Presbytis thomasi). Folia Primatologica 69, 77-80.
Tembrock, G. (1977). Tierstimmenforschung. Eine Einführung in die Bioakustik.
Wittenberg Lutherstadt: A. Ziemsen Verlag.
Tenaza, R. R. (1976). Songs, choruses and countersinging among Kloss' gibbons (Hylobates
klossi) in Siberut island, Indonesia. Zeitschrift für Tierpsychologie 40,
37-52.
Thalmann, U., Geissmann, T., Simona, A. and Mutschler, T. (1993). The indris of Anjanaharibe-Sud,
northeastern Madagascar. International Journal of Primatology 14, 357-381.
Thorpe, W. H. (1961). Bird-song. The biology of vocal communication and expression
in birds. Cambridge monographs in experimental biology No. 12. Cambridge: University
Press.
Tilson, R. L. (1977). Social organization of Simakobu monkeys (Nasalis concolor)
in Siberut island, Indonesia. Journal of Mammalogy 5, 202-212.
Tilson, R. L. (1981). Family formation strategies of Kloss's gibbons. Folia Primatologica
35, 259-287.
Tilson, R. L. and Tenaza, R. R. (1976). Monogamy and duetting in an Old World monkey.
Nature 263, 320-321.
van Gulik, R. H. (1967). The gibbon in China. An essay in Chinese animal lore. Leiden:
E.J. Brill.
Vogel, C. (1973). Acoustical communication among free-ranging common Indian langurs
(Presbytis entellus) in two different habitats of North India. American Journal
of Anthropology 38, 469-480.
de Waal, F. B. M. (1988). The communicative repertoire of captive bonobos (Pan
paniscus), compared to that of chimpanzees. Behaviour 106, 183-251.
Waser, P. M. (1977). Individual recognition, intragroup cohesion and intergroup spacing:
Evidence from sound playback to forest monkeys. Behaviour 60, 28-74.
Waser, P. M. (1982). The evolution of male loud calls among mangabeys and baboons.
In S. T. Snowdon, C. H. Brown and M. R. Petersen (Eds.), Primate communication (pp.
117-143). Cambridge: Cambridge University Press.
Wickler, W. (1980). Vocal dueting and the pairbond. - I. Coyness and partner commitment.
A hypothesis. Zeitschrift für Tierpsychologie 52, 201-209.
Wickler, W. and Seibt, U. (1982). Song splitting in the evolution of dueting. Zeitschrift
für Tierpsychologie 59, 127-140.
Ziegler, P. and Knobloch, J. (1968). Vergleichende und experimentelle Untersuchungen
zur Lautgebung der Hylobatini. Staatsexamensarbeit, Zoologisches Institut, Mathematisch-Naturwissenschaftliche
Fakultät, Humboldt-Universität, Berlin.
![]()