This section was published as:
Geissmann, T. 2000: Duet songs of the siamang, Hylobates syndactylus:
I. Structure and organisation. Primate Report 56: 33-60.
![]()
Abstract
1. Introduction
2. Animals, Material
and Methods
3. Description of
the Siamang Song Bouts
4. Discussion
5. Acknowledgements
6. References

Cover: Adult siamang pair (Hylobates syndactylus) during a duet song bout. Both siamangs on the photo are producing so-called boom notes (see article below). While producing these notes, the mouth is almost completely closed and the throat sac is being inflated. Notice the close proximity between the singers. Photo: Thomas Geissmann, Sept. 1998, Taman Safari Zoo, Cisarua, Java, Indonesia.
![]()
Abstract |
Siamangs (Hylobates syndactylus) produce loud and long song bouts which are mostly exhibited by mated pairs. Typically, mates combine their partially sex-specific repertoire in relatively rigid, precisely timed and complex vocal interactions to produce well-patterned duets. This study presents a detailed description of singing behaviour, repertoire, and song and duet organisation of several adult pairs of captive siamangs. A comparison of the findings of the present study with previously published descriptions of siamang duetting behaviour reveals that various authors have been using different terms when referring to the same vocal elements, while others have applied the same names to completely different elements. In addition, the organisation of both the song bout as a whole, as well as the great call sequence in particular, is more complex than has been believed previously.
1. Introduction |
2. Animals, Materials and Methods |
In order to describe siamang song organisation, 9 adult siamangs in 10 different
group constellations have been observed. The animals were kept at the Zoological
Garden of Zürich (Switzerland) and at the "Zoo Seeteufel" in Studen
near Biel (Switzerland). For the sake of brevity, these zoos will hereafter be referred
to as Zürich and Studen, respectively. Between November 1980 and February 1985,
tape-recordings of 134 song bouts were made in Zürich (n = 80) and Studen (n
= 54). As a supplement, a short sound film (8 mm) of siamang duets was made in Zürich.
Animals, their groupings, the number of song bouts tape-recorded of each grouping
and the recording dates are listed in Table 1.
| Animals | Zoo | Study period | Tape-recorded song bouts | |
| Begin | End | |||
| Ga+Na | Zürich | 29 Nov 1980 | 14 July 1981 |
17 |
| Bh(+Ch) | Zürich | 29 Nov 1980 | 12 Aug. 1981 |
23 |
| Ga | Zürich | 14 July 1981 | 12 Aug. 1981 |
4 |
| Bh+Ga | Zürich | 12 Aug. 1981 | 28 April 1982 |
33 |
| (Fa+Da)+Ch | Zürich | 12 Aug. 1981 | 20 Nov. 1984 |
2 |
| (Da+)Ch | Zürich | 20 Nov. 1984 | 22 Feb. 1985 |
1 |
| Vr | Studen | 6 July 1981 | 14 July 1981 |
10 |
| Na+Vr | Studen | 14 July 1981 | 24 Nov. 1981 |
34 |
| Bb+Ra | Studen | 6 July 1981 | 8 Oct. 1981 |
4 |
| Ra | Studen | 8 Oct. 1981 | 24 Nov. 1981 |
2 |
| Ko+Cr(+Li+Al) | Studen | 6 July 1981 | 24 Nov. 1981 |
4 |
| Total |
134 |
|||
The age classes proposed by Geissmann (1993) for captive gibbons and siamangs were
used: infants from 0 to 2 years of age; juveniles 2.1 to 4 years; subadults 4.1 to
6 years; adults more than 6 years.
Song organisation of the following 5 pairs, groups or solitary animals were analysed
in detail (group compositions and age classes given for the beginning of the study):
The adult pair Na+Ga at the Zürich Zoo consisted of the male Narong (Na), wild-born
about in 1967, and the female Gaspa (Ga), wild-born about in 1963. The pair was together
since July 1980.
The younger pair Bh+Ch at the Zürich Zoo consisted of the subadult male Bohorok
(Bh) and his juvenile sister Chandra (Ch), both captive-born offspring of Na+Ra,
and both hand-reared at Zürich Zoo. Bh was born on 23 June 1975 and was 5 years
and 5 months old at the beginning of this study. His sister Ch was born on 24 Dec.
1976 and was a 3 year 11 months old juvenile at the begin of this study. As her song
repertoire and the temporal structure of her song vocalisations were not yet fully
developed, the songs of this female were relevant to this study only because of her
vocal and other interactions with the other study animals at Zürich.
Vreneli (Vr) was an adult female at Studen. She was wild-born about in 1963 and has
remained solitary since both the offspring and her mate died in 1979.
The adult pair Bb+Ra at Studen consisted of the male Bobby (Bb), wild-born about
in 1958, and the female Ratana (Ra), wild-born about in 1963. This pair was together
since July 1980.
The family group Ko+Cr(+Li+Al) at the Studen Zoo consisted, at the beginning of this
study, of the adult male Kobi (Ko), the adult female Christeli (Cr) (both wild-born
about in 1963, and kept as a pair in Studen since about 1968), their juvenile daughter
(Li), and their infant son (Al). Cr gave birth to a healthy male on 28 Dec. 1981.
At the beginning of this study, the two siamang groups at Zürich Zoo consisted
of the pairs Na+Ga and Bh+Ch. On 14 July 1981 Na was transferred to Studen Zoo. In
Zürich, his former partner Ga was paired with Bh on 12 Aug. 1981. Because Bh's
sister Ch was not considered yet to be sexually mature, she was kept with two hand-reared
juvenile males (Fa and Da) after her separation from Bh, and with only one of them
(Da) after 20 Nov. 1984.
In Zürich, the siamangs were kept in two adjacent indoor-cages (base area: 16
m2 and 40 m2; height: 4 m). In summer, both siamang groups
were alternatively given access to a large outdoor cage (30 m2 x 4.6 m).
All cages were equipped with extensive bamboo scaffolding and ropes. Both groups
could hear (but not see) each other at any time.
In Studen, the original constellation consisted of one solitary female (Vr), one
pair (Bb+Ra) and one family group (Ko+Cr(+Li+Al)). On 14 July 1981, the adult male
Na arrived from Zürich and was kept as a pair with Vr. On 8 Oct. 1981, Bb died
of a Clebsiella infection.
During summer, all groups were kept in outdoor-cages (25 m2 x 2.5 m) equipped
with several horizontal stainless metal bars, ropes, and a wooden sleeping box. Two
of the cages stood close together at a sharp angle. The third cage (of the family
group) was located in a distance of more than 10 m. The sight from cage 3 to the
other two cages was somewhat reduced by shrubs and trees. During winter, the siamangs
were housed in a separate building. Two of the winter cages (18 m2 x 2
m, and 14 m2 x 2 m, respectively) were situated on the first floor and
stood about 3.5 m apart; these two groups could see each other. The winter cage of
the family group (6 m2 x 3 m) was situated on the ground flour and could
not be seen by the other two groups. As in Zürich, all groups could hear each
other during the whole year.
A note is defined as "any single continuous sound of any distinct frequency
(pitch) or frequency modulation, which may be produced by either an inhaled or exhaled
breath" (Haimoff, 1984, p. 335). Based on the acoustic characteristics, these
notes can be divided roughly into four groups or classes: booms, barks, ululating
screams and bitonal screams.
Note classes can be subdivided into different note types. There are no strict borders
between types of the same note class, and vocalising animals may gradually switch
from one type to another. On the other hand, no intermediate forms were found between
different note classes.
A phrase is a collection of several notes separated by short pauses of no more than
3 s duration and preferentially uttered in combination.
Larger song sections may differ from each other in the characteristic frequency of
some phrases, note classes, or types, and in the variability of the rules governing
note successions. These larger song sections are termed sequences (for an alternative
definition of the same song sections see Haimoff, 1981, 1984).
A song is what fulfils the criteria set forth by Thorpe (1961, p. 15): "What
is usually understood by the term song is a series of notes, generally of more than
one type, uttered in succession and so related as to form a recognisable sequence
or pattern in time", or, a song is a succession of phrases with non-random succession
probability ("Strophenfolgen mit nicht-zufälliger Folgewahrscheinlichkeit",
Tembrock, 1977, p. 33). Song bouts are separated from each other by an arbitrarily
defined interval of at least 5 minutes.
Some authors have referred to individual phrases within a song bout as "songs"
(e.g. Marshall & Sugardjito, 1986). I recommend using the term "phrase"
instead, in order to make it clear whether one is talking about a mere collection
of notes (usually shorter than 1 minute), or about a song bout (i.e. a collection
of phrases, with an overall duration of several minutes).
A duet occurs when one individual coordinates its vocalisations in time or type of
vocalisation with those of another individual (Seibt & Wickler, 1982; Wickler,
1974). Accordingly, a duet song is a song jointly uttered by two individuals and
coordinated in time or phrases. A concert occurs when a song is uttered in coordination
by more than two individuals, whereas a chorus is defined as a vocalisation bout
without song structure, which is jointly uttered by more than two individuals (Tembrock,
1974, p. 189).
Various authors have created different terminologies for siamang song vocalisations.
In order to facilitate comparison between the present study and earlier publications,
the following description of vocalisations also includes cross-references to terminologies
used by other authors.
3. Description of the Siamang Song Bouts |
Unless noted otherwise, the classification of siamang song vocalisations used in
this paper follows, and expands on, that of Lamprecht (1970) and Haimoff (1981).
This classification is chiefly based on the physical characteristics and the temporal
pattern, i.e. on structural and syntactical aspects (Marler, 1965; Tembrock, 1974).
The frequency and duration ranges of all types and classes of song vocalisations
summarised in Table 2, and a sonagram of each is shown in Figure 1.
| Class | Type | Adult song repertoire 1) | |
| Male | Female | ||
| Booms | Grunts | + | + |
| Short booms | + | + | |
| Long booms | + | + | |
| Ascending booms | + | (+) | |
| Barks | Short fast barks | + | - |
| Short slow barks | + | - | |
| Long barks | - | + | |
|
Ululating scream |
+ | (+) | |
|
Bitonal scream |
+ | - | |
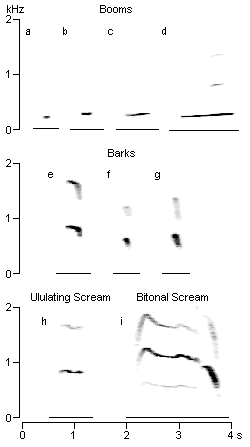
Figure 1: Sonagrams of note classes typically produced during siamang song bouts, including booms (a = grunt; b = short boom; c = long boom; d = ascending boom), barks (e = long bark; f = short fast bark; g = short slow bark), ululating scream (h), and bitonal scream (i).
The frequency and duration ranges of all types and classes of song vocalisations
are summarised in Table 3. For all siamang song vocalisations, the fundamental frequency
is situated between 0.13 and 1.47 kHz. Only few such measurements have previously
been published (Haimoff, 1983b, Lamprecht, 1970, Schröpel, cited in Tembrock,
1974, West, 1982). They largely support the data presented in Table 3. The frequencies
published by Barkell (1988), however, are all situated between 1 and 7.5 kHz. These
values are not compatible with those presented here; apparently, Barkell measured
harmonics, not the fundamental frequency.
| Class | N 2 | Duration (s) | Fundamental frequency (kHz) | ||||||
| Type | Minimum | Maximum | Modulation Range | ||||||
| Median | Range | Median | Range | Median | Range | Median | Range | ||
| Booms | |||||||||
| Grunts | 14 | 0.15 | 0.10-0.19 | 0.167 | 0.127-0.216 | 0.282 | 0.231-0.317 | 0.109 | 0.047-0.190 |
| Short booms 1 | 11 | 0.09 | 0.05-0.11 | 0.301 | 0.259-0.346 | 0.371 | 0.361-0.410 | 0.074 | 0.060-0.110 |
| Short booms 2 | 15 | 0.21 | 0.16-0.31 | 0.257 | 0.215-0.307 | 0.357 | 0.304-0.410 | 0.103 | 0.071-0.132 |
| Short booms all | 26 | 0.18 | 0.05-0.31 | 0.286 | 0.215-0.346 | 0.369 | 0.304-0.410 | 0.079 | 0.060-0.132 |
| Long booms | 12 | 0.50 | 0.28-0.69 | 0.225 | 0.203-0.270 | 0.314 | 0.279-0.491 | 0.084 | 0.053-0.222 |
| Ascending booms | 14 | 0.55 | 0.46-0.89 | 0.224 | 0.194-0.239 | 0.335 | 0.276-0.377 | 0.111 | 0.064-0.153 |
| Barks | |||||||||
| Short fast barks (SFB) | 14 | 0.14 | 0.11-0.15 | 0.375 | 0.333-0.463 | 0.808 | 0.742-0.869 | 0.405 | 0.361-0.488 |
| Short slow barks (SSB) | 19 | 0.13 | 0.10-0.16 | 0.385 | 0.311-0.438 | 0.720 | 0.670-0.831 | 0.346 | 0.289-0.467 |
| Long barks (LB) | 12 | 0.25 | 0.17-0.33 | 0.573 | 0.493-1.135 | 0.798 | 0.691-1.472 | 0.214 | 0.120-0.371 |
| Ululating scream (US) | |||||||||
| US I | 12 | 0.61 | 0.38-0.72 | 0.648 | 0.508-0.808 | 0.913 | 0.773-0.978 | 0.221 | 0.113-0.470 |
| US II | 14 | 0.74 | 0.32-1.25 | 0.642 | 0.340-0.816 | 0.878 | 0.807-0.956 | 0.226 | 0.093-0.500 |
| Bitonal scream (BS) | |||||||||
| BS, first phase | 13 | 1.05 | 0.67-1.40 | 0.463 | 0.357-0.486 | 0.598 | 0.486-0.824 | 0.128 | 0.076-0.349 |
| BS, second phase | 13 | 0.48 | 0.30-0.68 | 0.477 | 0.415-0.780 | 0.892 | 0.766-1.008 | 0.377 | 0.120-0.510 |
Equivalent terms: "Heullaute" (Lamprecht, 1970); "howling" (Schröpel,
cited in Tembrock, 1974); "booms" (Chivers, 1974; Fox, 1977; Haimoff, 1981).
Booms are "ooo"-like notes of low frequency. They are usually produced
with the mouth closed and maximally inflated throat sac or during inflation of the
throat sac. Longer booms (see below) are mostly produced while the vocalising animal
is stationary (in sitting or hanging position). On the basis of their duration, frequency
modulation and association with other vocalisations, a number of different types
of booms can be differentiated. Because all possible transitional forms occur, booms
cannot always be attributed to a single type. This finding contrasts with the view
offered by Haimoff (1983a, p. 68), who defined the various types of booms as completely
segregated vocalisations without overlap. The following four types of booms are recognised
here: grunts, short booms, long booms, and ascending booms, which may occur in different
contexts.
a) Grunts (Fig. 1a):
Grunts (Haimoff, 1983a, p. 73; "glunks", Fox, 1977, p. 453) are short,
soft vocalisations. Although Bricknell (1992) and Chivers (1974, p. 237) described
them for females only, grunts are uttered by siamangs of either sex. The throat sac
is only minimally inflated during grunts. In the sonagrams, grunts resemble short
booms, but their intensity is much lower. Grunts are produced at the beginning of
a song bout. Grunts are also uttered while feeding (Chivers, 1976, p. 119; Fox, 1977,
p. 442), when the siamang see the keeper arriving with food, or in early stages of
an alarm reaction ("soft grunts", Chivers, 1976, p. 119).
b) Short booms (Fig. 1b):
Short booms usually occur as single notes, in series, as the second note in a pair
of booms (corresponding to "diphasic"-booms in Chivers, 1974), or immediately
prior to a bark, an ululating scream, or a bitonal scream. Short booms differ in
their duration and frequency characteristics depending on context and several distinct
types of short booms could possibly be recognised. For instance, short booms occurring
during very rapid barking (SFB) are much shorter and show less frequency modulation
than booms occurring immediately prior to a ululating scrteam II (see "short
booms 1" and "short booms 2", respectively, in Table 3).
c) Long booms (Fig. 1c):
These booms occur as single notes, as the first note in a pair of booms, and prior
to long barks. Some females, however, produce only short booms in the latter context.
d) Ascending booms (Fig. 1d):
Ascending booms (Haimoff, 1981, 1983a) are long booms of an increasing frequency
modulation. They occur almost exclusively in the first position within pairs of booms.
These pairs occur in three different contexts: 1. as single elements within the song,
2. as the first note in a pair of booms immediately prior to an ululating scream,
or 3. during the initial phase of a great call sequence prior to the bitonal scream
(see below). Whereas short and long booms are produced by male and female siamangs,
Haimoff (1981) described ascending booms only for males. As a rule, this type of
vocalisation is, in fact, produced by males only, but some of the females studied
here frequently produced ascending booms (e.g. one female each in Doué-La-Fontaine,
Studen, Taman Safari/Cisarua and Twycross).
The use of bi-phasic notes (alternate production of exhalation and inhalation sounds)
during the song probably represents a primitive characteristic for both male and
female gibbon vocalisations (Geissmann, 2000). Previous authors disagreed on whether
all notes in the song of adult siamangs are produced during exhalation (Haimoff,
1981; Lamprecht, 1970) or whether some of them (the boom notes) are produced during
inhalation (Tembrock, 1974, p. 178; Welch, 1911, p. 358). During the boom notes,
the throat sac is inflated; this could be occurring through either exhalation or
inhalation. Close observation of several siamang pairs during the present study indicates,
however, that at least some booms may be produced during inhalation. The visible
contractions of ventral rump muscles of vocalising siamangs suggest that the alternation
of barks and booms during the acceleration of the female great call phrase consist
of an alternation of exhalation (barks) and inhalation (booms).
This interpretation is further supported by observation of an adult female hybrid
between a Mueller's gibbon and a siamang (H. muelleri x H. syndactylus).
Like most gibbon species, this female hybrid had no visible throat sac. During the
acceleration of its great call, the female produced bi-phasic notes clearly consisting
of sounds alternatingly produced during exhalation (low frequency) and inhalation
(high frequency). This finding is relevant, because species-specific characteristics
of gibbon songs are largely inherited (Brockelman & Schilling, 1984; Geissmann,
1984, 1993). As Mueller's gibbons do not produce inhalation notes during their great
calls (Geissmann, 1993), the occurrence of this characteristic in the hybrid suggests
that it was inherited from the siamang parent and provides indirect support to the
hypothesis that inhalation notes typically occur in the siamang great call. It remains
unknown, however, whether booms other than those emitted during the great call acceleration
are inhalation notes.
Equivalent terms: "Bellaute" (Lamprecht, 1970); "barking sounds"
(Schröpel, cited in Tembrock, 1974); "barks" (Chivers, 1974; Fox,
1977; Haimoff, 1981).
Together with booms, barks comprise the most frequent vocalisations in the siamang
song. Barks are produced as single notes or in series but almost every bark is preceded
by a short boom. During barks, the mouth is opened moderately and the teeth remain
hidden behind the lips. As in booms, the various types of barks are parts of a graded
system and are linked by barks of intermediate characteristics.
a) Long barks (Fig. 1e):
This type of vocalisation is produced by females only (although this has been questioned
by Fox, 1977) and is, as a rule, uttered in accelerated series. The acceleration
is created by a gradual reduction in the duration of the barks as well as of the
intervals between the barks. With increasing speed and intensity, the long barks
continuously change into short, fast barks (see below, type b). In parallel with
the acceleration, the booms which precede each bark become shorter and rise in frequency.
This rise ("raised bass", Marshall and Marshall, 1976) is not equally pronounced
in all females; in some animals it can hardly be heard. Long barks are called "high
barks" by Maples et al. (1989). During this vocalisation, females usually remain
stationary or move around only slowly.
b) Short fast barks (Fig. 1f):
In contrast to long barks, these other types of barks are produced by animals of
either sex. Short fast barks often develop out of long barks during the acceleration
described above. Independent series of short fast barks also occur frequently during
the song. During these very fast strings of vocalisations, the mouth of the calling
animal is not completely closed between the single barks. During series of short
fast barks, siamangs frequently exhibit rapid, vigorous locomotion, hence the name
"locomotion call" proposed by Haimoff (1981). In contrast to this observation,
Benchley (1942, pp. 24, 27) reports that siamangs sit still during their songs.
c) Short slow barks (Fig. 1g):
Like the previous type of barks, short slow barks are produced by animals of either
sex. This bark can be uttered as a single sound or in series, often with irregular
intervals. When producing short slow barks, siamangs usually remain stationary or
move around slowly. This type of vocalisation may continuously develop out of short
fast barks (type b) or, less frequently, out of long barks (type a). Short slow barks
are probably identical to the "barking sound of medium length" described
by Schröpel (cited in Tembrock, 1974, p. 187).
Equivalent terms: "Jauchzer" (Lamprecht, 1970); "rejoicing" (Schröpel,
cited in Tembrock, 1974); "cry" (Fox, 1977); "ululating scream"
(Haimoff, 1981); "undulating scream" (West, 1982).
This long note of slightly decreasing frequency (Fig. 1h) is produced with a fully
inflated throat sac and a widely opened mouth. The teeth are not bared, however.
This sound is never uttered in series. The vocalising animal is stationary or moving
around slowly.
The ululating scream is nearly always produced in association with other types of
vocalisation, thus producing a short combination of notes corresponding to the term
"phrase" in Haimoff (1984) or the term "Motiv" in Thielcke (1961).
Ululating screams of all animals of this study are preceded by a pair of booms, the
first of which is an ascending boom, the second a short boom. The second boom precedes
the scream so closely that no interval is perceived by the observer, similar to the
situation in many booms preceding barks. Following the ululating scream, the siamang
almost invariably utters a brief series of short fast barks.
Individual characteristics are particularly striking in the ululating scream phrase
and become manifest in the duration of the interval between the ascending and the
short boom (or between the ascending boom and the ululating scream, respectively),
in the number of short barks following the ululating scream, and in the frequency
modulation of the ululating scream itself. Whereas these parameters clearly varied
individually, they remained relatively constant in the song bouts of each animal.
For example, one of the adult males of this study never produced the series of short
barks after his ululating screams. Similar individual characteristics were also mentioned
by Lamprecht (1970, p. 197) for some of his study animals.
Two variants of ululating screams are recognised in this study: (1) ululating screams
occurring in the middle of the great call sequence (US-I), and (2) ululating screams
occurring at the beginning, at the end or outside of the great call sequence (US-II).
The two variants are more or less distinct - depending on the animal - in the duration
and the frequency modulation of the scream note, but also in the number of short
barks following the scream. In some males, no clear difference in the acoustical
structure of the two screams was obvious.
Many individuals terminate the US-II phrase by an additional pair of booms. Like
the pair of booms which introduces the phrase, this final pair of booms consisted
of an ascending boom followed by a short one in the study animals; only the adult
male Na frequently omitted the short boom. In the adult male of Haimoff's (1981)
study, the ascending boom was followed by a long boom instead of a short one.
In the studies of Fox (1977), Haimoff (1981) and Lamprecht (1970), only males were
observed to produce ululating screams. In the majority of the animals studied I observed,
this rule appeared to hold true. Several females, however, regularly produced ululating
screams in their songs, always of the variant II (e.g. one female each in Doué-La-Fontaine,
Studen, Taman Safari/Cisarua, Twycross and Zürich). These screams did not fall
outside the range of variability of male ululating screams and also included the
whole ululating scream phrase described above. The female observed by Schröpel
(cited in Tembrock, 1974) also appears to have used this class of vocalisation frequently.
The vocalisation termed î'ululating' callî in Barkell (1988, p. 3) is completely
different from the one described above. Barkell's description of the sound and the
degree of the vocalising animal's mouth aperture perfectly fit, in fact, another
class of vocalisation which is discussed below: the bitonal scream.
Equivalent terms: "Schrei mit anschliessendem Jauchzer" (Lamprecht, 1970);
"climax" (Fox, 1977); "bitonal scream" (Haimoff, 1981).
This vocalisation consists of two different phases (hence the name "bitonal")
which differ in their frequency and which follow each other with only a minimal time
interval. By the definition used in this paper, they are two different notes. Because
both are consistently linked with each other and because of the transition mechanism
discussed below, it appears logical to combine them both under the same class of
vocalisations.
The first phase of the bitonal scream consists of a drawn out note of a duration
of 0.67-1.40 s, and of relatively constant frequency. During this sound, the throat
sac is maximally inflated, and the mouth is opened widely, with bared teeth. The
second phase is quite variable in frequency modulation. As a rule, it is considerably
shorter (0.30-0.68 s) than the first phase, and usually of decreasing frequency.
The throat sac is not in maximal inflation, the teeth are not bared. Bitonal screams
are never uttered in series.
Lamprecht (1970, p. 188) was the only author to treat the two phases as two distinct
vocalisations. He called the first phase "Schrei" and considered the second
phase as being identical to the ululating scream. In most siamangs, however, the
second phase of the bitonal scream differs in its frequency structure from the ululating
scream and is usually shorter than the latter (see Table 3 and Figure 1).
To human observers, the first phase of the bitonal scream always appears to be of
lower frequency than the second. Interestingly, both Lamprecht (1970) and Haimoff
(1981, 1983b) observed just the opposite on their sonagrams: The fundamental frequency
of the first phase appeared to be higher (0.96-1.25 kHz) than that of the second
phase (0.42-1.00 kHz). Haimoff (1981, 1983b, 1984) suggested that the real fundamental
frequency of the first phase is filtered away or suppressed by anti-resonance and,
hence, only the second harmonic was visible on sonagrams. This effect is, apparently,
neutralised when the throat sac is deflated during the second phase of the bitonal
scream.
During this study, however, high quality tape-recordings of songs of various siamang
males (e.g. Na and Bh) made it possible to see a very weak additional frequency band
below what has previously been believed to be the fundamental frequency of the first
phase of the bitonal scream (the lowest of the 3 bands visible in Fig. 1i). This
weak band appears to be the real fundamental frequency of this phase. Its intensity
is considerably lower than that of the first harmonic, suggesting that the basal
frequency is at least partially filtered away. The high frequency attributed by Lamprecht
(1970, p. 188) and West (1982, p. 16) to the first phase of the bitonal scream (1.13-1.21
kHz and 1.08-1.23 kHz, respectively), refers, therefore, to the first harmonics (as
correctly suggested by Lamprecht, 1970), and not to the fundamental frequency. According
to the measurements carried out in the present study, the real fundamental frequency
of the first phase of the bitonal scream is situated between 0.36 and 0.82 kHz and
is clearly lower than that of the second phase (0.42-1.00 kHz).
The captive male siamang observed by Bricknell (1992) produced bitonal screams, in
which no first phase was visible on the sonagrams at all, and with the second phase
"starting at a frequency of 1.8 kHz and ending at 1.3 kHz". This range
is situated above that of all males heard during the present study (0.42-1.00 kHz).
The bitonal screams uttered by that male appear to be atypical.
The bitonal scream is almost always combined with locomotion. Usually, the calling
animal moves only a short distance, by swinging itself to another branch or sitting
place; sometimes the animal merely changes its position.
Similar to the ululating scream, the bitonal scream is associated with other vocalisations,
thus forming a particular phrase. The phrase begins with a short boom, or - in some
individuals of this study - a pair of booms consisting of an ascending boom followed
by a short boom. After the short boom, the animal utters its bitonal scream, which
is in some animals followed by a number of short fast barks. The bitonal scream phrase
is, therefore, similar to the ululating scream phrase in its structure. Here, too,
some individual characteristics are evident. They include the frequency modulation,
the intensity of the scream, and the number, structure and interval duration of its
accompanying phrase elements. In this study, the two males Bh and Na produced a short
series of 1-4 short fast barks immediately after the scream, whereas no barks were
uttered after the bitonal screams of another male (Bb). These particular barks also
appeared to be missing in the males observed by Haimoff (1981), Maples et al. (1989)
and West (1980), as well as in two of four males in Lamprecht's (1970, p. 197) study.
One of the males of this study (Bb) always produced the first phase of its bitonal
scream so softly, that it was sometimes difficult to hear when the male was duetting
with a female. The second phase of this male's bitonal scream, however, was of the
normal intensity. In exceptional cases, all males can omit the second phase of the
bitonal scream. One of the males observed by West (1980, p. 12) in Sumatra always
produced only the first phase.
It is not clear, whether the two phases of the great call are both produced during
exhalation, as suggested by Haimoff (1981, 1983b), or whether one phase is an exhalation
note.
The bitonal scream is only known from male siamangs. Rühmekorf (1963) described
bitonal screams of a female siamang, but, at that time, the sex of this animal could
not be determined with certainty, but later, the animal was identified as a male
(Rühmekorf, pers. comm.).
3.2 Song Bout Organisation |
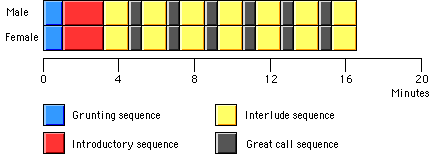
Figure 2: Stylised diagram illustrating the sequential nature of the duet song bout produced by adult siamang pairs (after Haimoff, 1984, supplemented).
At the beginning of a song bout, siamangs usually utter only soft grunts for a few
minutes (Figures 2 and 3). These vocalisations have been mentioned in earlier studies
(Bricknell, 1992; Chivers, 1974, p. 237; Fox, 1977, p. 453; Haimoff, 1983a, p. 73;
West, 1982, p. 6), but were not regarded as being part of the song bout and the organisation
of these series of grunts has apparently not been analysed.
Because the grunts typically occur at the beginning of the siamang song bout and
because they essentially appear to be soft booms (which are typical song elements),
this study regards the grunts as part of the song. It is unknown, however, whether
mates coordinate their grunts (i.e. whether they are duetting during this sequence).
The grunting sequence may have been neglected in earlier studies of siamang singing
behaviour, because a similar sequence does not appear to exist in the duet songs
of other gibbon species.
Although some authors heard grunts only being uttered by females (Bricknell, 1992;
Chivers, 1974), the males of the present study also participated in the grunting
sequences. It should be mentioned that the grunting sequence may sometimes be missing
and the siamangs may directly start their song with the barks and booms of the introductory
sequence (see below). In other instances, one animal was observed to grunt alone
for some time before it was joined by its partner. Grunts may also occur in other
contexts (section 3.1.1). In general, grunts appear to indicate that the calling
animal is moderately excited.
The beginning of the proper song bout (i.e. after the grunting sequence) consists,
according to the traditional view, of the îintroductory sequenceî (Haimoff, 1981).
Like the grunting sequence, the introductory sequence is produced only once at the
beginning of a song bout. It consists mainly of short single barks, short series
of barks, and short booms (and occasionally other types of booms). During the sequence,
mated siamangs appear to synchronise their barks or bursts of barks (Figure 3).
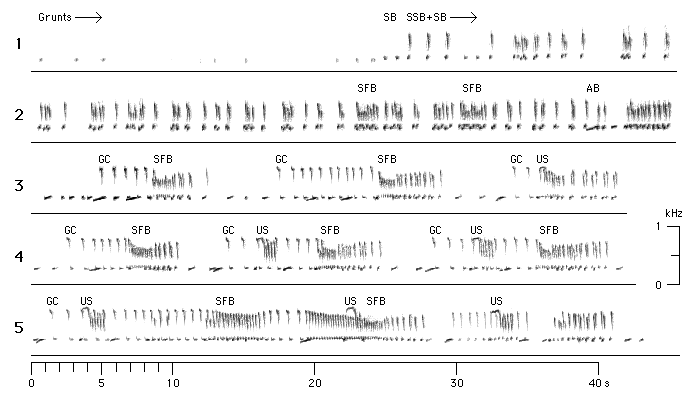
Figure 3: The early stages of a duet song bout of pair Bh+Ga. The sonagrams represent a continuous sequence, beginning with the grunting sequence (line 1). At the end of the sequence (at about 25 s on line 1), the grunts change into booms (SB). The first barks (SSB) mark the end of grunting sequnce and the beginning of the introductory sequence (at about 26.5 s on line 1). The first occurrence of an ascending boom (AB) indicates the end of the introductory sequence and the beginning of the first interlude sequence (at about 39 s on line 2). The first great call sequence begins on line 3 (GC). Lines 3-5 show the first 7 great call sequences of the song bout, each followed by an interlude sequnce. The female aborts each great call before completion except the last one, and all great call sequences lack the full set of male contributions which is found in typical great call sequences later in the song bout, i.e. after the first bitonal scream of the male (see Fig. 5). Abbreviations: AB = ascending boom (only the first one of this song bout is labelled in the sonagram); GC = start of a female great call; SB = short booms; SFB = short fast barks; SSB = short slow barks; US = ululating scream of the male.
Equivalent terms in previous publications: "Zwischenphase" (Lamprecht,
1970); "organizing sequence" (Haimoff, 1981); "interlude sequence"
(Haimoff, 1988).
The interlude sequence has a typical duration of 5-60 s; in exceptional cases it
can be extended to up to several minutes. The organisation of this sequence is very
variable (Figures 3 and 5) and is combined of booms (types a-c), barks (types b and
c) and ululating screams (US-II). In this study, the term "interlude sequence"
refers to a shorter song segment as compared to the definition used in Haimoff (1981)
and Lamprecht (1970): After each great call, males (and sometimes females, too) utter
a US-II phrase. Because the interval between the phrase and the great call has an
individually constant duration, the US-II phrase is considered here to be part of
the great call sequence; whereas Haimoff (1981) and Lamprecht (1970) treated it as
part of the interlude sequence.
The organisation of the interlude sequence can show considerable differences between
song bouts of the same pair. The interlude sequences may include many elements of
a particular type of vocalisation in one song bout, but only a few in another song
bout. Many different note combinations have been observed to occur during interlude
sequences. Haimoff (1981, p. 136) suggested that these combinations occurred at random,
but this has not yet been analysed.
Siamangs frequently exhibit locomotion during the interlude sequence. Phases of rapid
locomotion occurred especially during the short fast barks (type b). These series
of barks were often produced in synchrony by mated pairs, but sometimes females accompany
the males' short fast barks with short slow barks only. Every bark note is usually
introduced by a short boom. In addition, pairs of booms are frequently produced during
the interlude sequence. As a rule, the first boom of the pair is an ascending one
(if produced by a male), or a long boom (if from a female), and the second boom is
a short one. The ululating screams are usually uttered in combination with other
notes so as to form a US-II phrase (as described above).
Equivalent terms in previous publications: "Duett" (Lamprecht, 1970; "great
call sequence" (Haimoff, 1981).
The great call sequence has a duration of about 20-50 s. It includes the great call,
which consists of two series of accelerated barks produced by the female. At certain
points in each series of barks, the male inserts particular combinations of vocalisations
(Lamprecht, 1970). The organisation of a typical great call sequence is shown in
Figure 4. The term "great call" was originally coined by Boutan (1913,
p. 33: "grand chant d'excitation") for a particular series of vocalisations
produced by a female white-cheeked gibbon (H. leucogenys leucogenys).
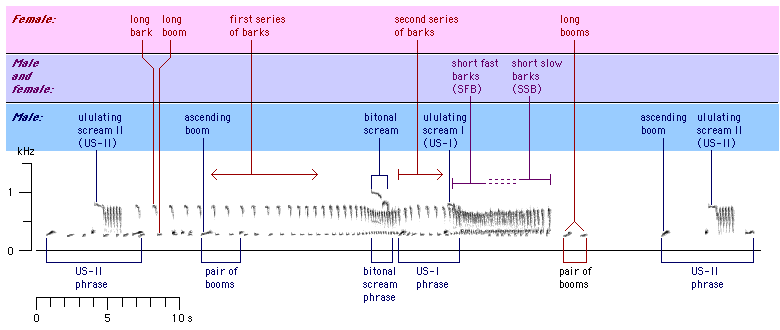
Figure 4: Organisation of a typical great call sequence of pair Na+Ga. Selected notes or groups of notes are individually indicated.
Great calls are associated with of female siamangs only, at least as far as adult
animals are concerned. Barkell (1988, p. 3) reports great calls of male siamangs,
but her (unfortunately very indistinct) sonagram of that phrase (her Figure 3) rather
resembles the final half of an ululating scream phrase, whereas her description of
the "'ululating' call" clearly can be identified as a bitonal scream.
The great call sequence is a combination of vocalisations of all classes. Some vocalisations
are produced exclusively during this sequence, such as the long barks (type a), the
ululating scream variant I, and the bitonal scream.
A series of booms ends most interlude sequences and marks the beginning of the next
great call sequence. The males of this study usually produced pairs of booms during
this part of the song bout. These booms resembled those produced during the US-II
phrase: an ascending boom followed by a short boom. Indeed, the booms at the transition
from the interlude to the great call sequence are frequently part of a US-II phrase.
In many cases, the male's short boom is immediately followed by an ululating scream,
while the female has already started the long barks which are part of the great call.
The ululating scream leading from the interlude sequence to the great call sequence
can be seen in Figures 4 and 5, but also on the sonagrams in Lamprecht (1970, p.
191) and Marshall and Marshall (1976, p. 236). The male studied by Haimoff (1981,
p. 136f) produced pairs of booms in this part of the song bout, but these pairs of
booms consisted of an ascending boom followed by a long boom, instead of a short
one.
The pairs of booms, as well as the ululating scream which often follows them, show
a very constant temporal relationship to the female's great call. A constant timing
is typical of song elements produced during great call sequences. Although either
the booms and the "initial" US-II phrase are not always produced at the
beginning of the great call, they are attributed to the great call sequence in this
study.
At the beginning of a great call sequence, the duetting siamangs are stationary (i.e.
either sitting or hanging by their hands). The female begins the great call with
a rhythmic series of long barks. Each of her barks is introduced by a boom (usually
long booms). This first series of barks is continuously accelerated by shortening
the duration of the barks and the intervals between them. In synchrony with the female's
barks, the accompanying booms are shortened. During the long barks, the male typically
produces only the ascending boom which announces his bitonal scream. This boom, however,
may be absent, as is usually the case in the songs of the male Na. During the acceleration,
the long barks continuously change to short fast barks. Near the end of the first
series of barks - at the climax of the acceleration - the male inserts its bitonal
scream.
The bitonal scream is usually produced during locomotion: In most cases, the male
swings to another place in the cage. The female, too, becomes more active and begins
to brachiate around with increasing speed of her barks. Occasionally, both animals
may confine themselves to a change of their positions. The order of these events
is variable: in most cases, it is the male who starts locomoting; this is followed
by his bitonal scream, and finally, the female begins to brachiate. A frame-by-frame
analysis of the film recordings demonstrated, however, that sometimes the female
starts to locomote before the male, unlike the siamang pair observed by Haimoff (1981,
p. 139).
After its bitonal scream, the male often utters a few short barks and then immediately
begins with the US-I phrase: It produces the pair of booms (ascending boom, then
short boom) which introduces the ululating scream.
With the end of the male's bitonal scream, the female begins a second accelerated
series of barks, by which time both animals have returned to a stationary position.
Again, the second series of barks begins with long barks, which gradually change
to short fast barks during the acceleration. This series of barks, however, begins
with a faster rhythm, and the acceleration sets in earlier than in the first series.
At the climax of the second series, the male sets in with its ululating scream. It
finishes the US-I phrase and then immediately joins the female in its short fast
barks. In this phase of the great call sequence, both animals exhibit the fast, vigorous
brachiation display ("Umhertoben", Lamprecht, 1970, p.193) which is typical
for the "locomotion call". In a pair observed by Maples et al. (1989),
the female hardly participated in this locomotion display.
After a few seconds, the pair's series of short fast barks is replaced by short slow
barks, and finally, the barking stops completely. Both animals then produce a few
booms. Those of the female usually include, among others, a long boom; those of the
male include a pair of booms which represents the introduction of the US-II phrase
which concludes the great call sequence.
Some females tend to accompany the ululating scream of their mate with a few short
slow barks, the occurrence of these notes as well as the duration of the interval
since the preceding vocalisations are very variable. The end of the great call sequence
is, therefore, arbitrarily defined to be situated at the end of the male's US-II
phrase. The concluding US-II phrases of the male were lacking in 19 out of 20 great
call sequences of the pair "Ulli" and "Tilly" at Frankfurt Zoo
(tape-recorded on 19 and 20 Sept. 1981). In this respect, the great call sequence
of the Frankfurt male appears to differ from that of other siamangs. The US-II phrase
is produced by all other males whose songs I was able to analyse, as well as by some
of the females (e.g. Vr).
The great call sequence is a complex, ordered chain of interactions between the mates:
At certain predictable points in the sequence, each animal utters certain types of
vocalisations in a particular temporal pattern, and exhibits particular types of
locomotion. The order of these interactions follow the same pattern in all pairs
of this study.
Some adult siamangs were observed to hold a hand or an arm in front of their mouth
(or in front of the throat sac) during certain of their song vocalisations. The male
"Ulli" (Frankfurt Zoo) did this during many of his bitonal screams, US-I
and -II phrases and series of short fast barks. The females "Tilly" (Frankfurt),
Ga (Zürich) and Ra (Studen), typically exhibited this behaviour during their
great calls (i.e. during both series of long barks). This particular behaviour has
also been described by Bricknell (1992), Fox (1977), Hess-Haeser (1971), Lamprecht
(1970), Rühmekorf (1963) and Schirmeier (1966). Its functional significance
is unclear.
The male's main vocal contributions during the female's great call consist of the
bitonal scream phrase, followed by the US-I phrase (see Figure 4). This is the characteristic
order of vocal events which has been described first by Lamprecht (1970). It has
later been confirmed by Haimoff (1981), Maples et al. (1982) and West, 1982, p. 13ff)
and also corresponds to the typical sequence I heard in 17 additional siamang pairs
in various zoos. Only Fox (1977) reported that the siamang males in her study produced
their screams in the opposite order of the one described by Lamprecht (1970). Her
description of the great call sequence makes it obvious that she had applied Lamprecht's
term "Schrei" (i.e. the bitonal scream) to the "Jauchzer" (i.e.
the ululating scream), and vice versa; It can, therefore, be concluded that both
authors had observed the same order of male vocalisations during the great call sequence.
The great call sequence is variable; numerous variations of the typical pattern are
possible. These variations occur mainly at the beginning of a song bout, and the
first great call sequences are frequently aborted (Haimoff, 1981, 1988).
The first attempts of great call sequences during a song bout usually consist of
a few long barks of the female. The number of barks progressively increases during
subsequent great call sequences. The early series of long barks are not accelerated
and are frequently not accompanied by the typical male vocalisations. During the
early series of long barks, the male may not vocalise at all, or confine his contribution
to short fast barks. In subsequent great call sequences, US-II contributions occur
with increasing frequency. In later great call sequences, the male produces his introductory
vocalisations, the long barks of the female are produced in accelerated series, and
several series are occasionally sung in succession. Finally, the typical male contributions
occur during the climaxes (i.e. bitonal scream and US-I phrase).
As shown above, the great call sequence develops at the beginning of the song bout
from single long barks to the full complex sequence by a progressive addition and
elaboration of components. This development does not necessarily show a linear course.
Occasionally, the animals may fall back from a relatively complex stage of sequence
development to a simpler form and recapitulate the development. Similar fluctuations
have been described for the development of the frequency-modulated male phrases of
the yellow-cheeked gibbon (H. gabriellae) (Goustard & Demars, 1973, p.
181).
Once the great call sequence is fully developed, it remains essentially constant
until the end of the song bout (Figure 5), although untypical or aborted sequences
may occasionally occur.
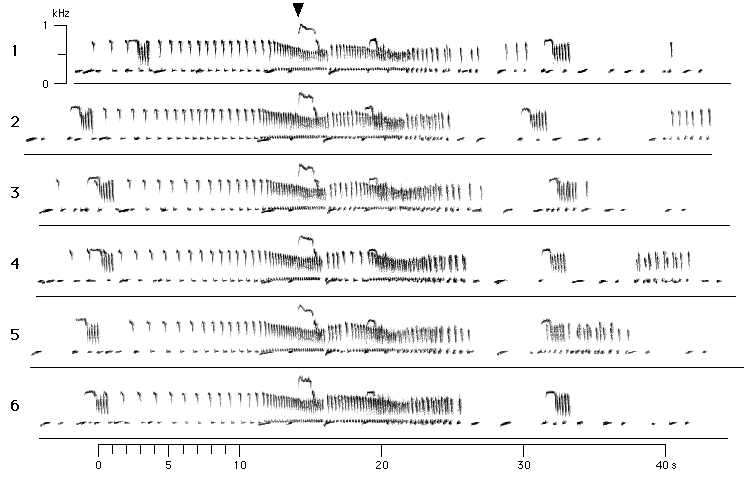
Figure 5: The first 6 great call sequences after having reached their stable form (after the first bitonal scream of the male) and the 6 subsequent interlude sequences of a song bout of pair Bh+Ga. The sonagrams represent a continuous sequence immeadiately following the one shown in Figure 3. Each line begins with a great call sequence and ends with an interlude sequence. Sonagrams are aligned by the bitonal screams (arrow) of the male Bh.
Individual-specific characteristics in the structure of single vocalisations have
been mentioned above. In addition to these, some individuals may also be recognised
by the note combination used during the great call sequence. Males may differ in
the number of short barks produced during an ululating scream phrase, females may
differ in the duration of their series of long barks, the speed of barking at the
beginning of these series, or the extent of their acceleration etc. (for additional
examples see Chivers, 1974, p. 238ff; Fox, 1977, p. 458f; Lamprecht, 1970, p. 197;
West, 1982, p. 13ff).
The various vocalisations of the great call sequence are not only uttered in a constant
order, but also with constant intervals between the notes. These intervals show individual-specific
durations. For instance, the means intervals between the initial long barks of great
calls produced by Vr (0.76 s) were about twice as long as those produced by Ra (0.34
s; p < 0.001; Mann-Whitney U Test). In the US-I phrase of Na, the interval between
the ascending boom and the ululating scream had a mean duration of 3.2 s, in Bh it
was 2.6 s (p < 0.01; Mann-Whitney U Test). Additional examples of individual-specific
intervals can be found in Geissmann (1999), Lamprecht (1970, p. 195) and West (1982,
p. 22).
Vocalisations of duetting animals are coordinated in time and type of vocalisation.
Mated siamangs not only synchronise the beginning and end of their song sequences,
but also exhibit a complex structure of vocal interactions within the sequences.
This coordination includes the types of vocalisations used by both animals, as well
as time intervals in the vocal interactions. In order to support the constant relationship
between their vocalisations, both mates must synchronise their vocalisations from
time to time. Lamprecht (1970) discovered at least four points where this happens
within the great call sequence. Haimoff (1981) suggested that the male's pairs of
booms at the beginning of the great call sequence initiate the beginning of the female's
great call, and that similar pairs of booms uttered during the female's first series
of long barks may initiate the acceleration of these barks. Pair-specific characteristics
also exist in the vocal interactions of mated siamangs (Lamprecht, 1970). At least
some of these pair-specific characteristics are not just identical with individual-specific
song characteristics, but are created by each siamang adapting its song to that of
its partner (Geissmann, 1999).
Incomplete or atypical great call sequences occurred in all siamang pairs of this
study. If one partner produced an atypical contribution to a great call sequence,
the sequence was often either aborted, or continued in a way that the atypical contribution
was corrected or compensated for. An example of this is presented here: If the male
inserted his bitonal scream before the acceleration of the female's first series
of barks, he would sometimes abort his premature contribution in mid-scream, wait
for the acceleration of his partner's barks, and then insert a second scream in the
proper place. As an alternative way of correcting the male's premature insertion
of a bitonal scream, the female sometimes quickly caught up by immediately producing
her acceleration in a foreshortened fashion, beginning a second series of barks and
accelerating it so soon that the male could insert the ululating scream-I in the
typical interval after his bitonal scream. Similar observations on siamang song variation
and correction have been reported by Haimoff (1981, 1988) and Maples et al. (1989)
and are also known of other gibbon species (Haimoff, 1984, 1988).
3.3 Alarm Call Bouts |
4. Discussion |
5. Acknowledgements |
6. References |
![]()