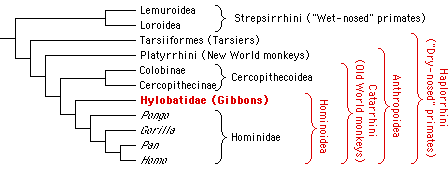
It is generally accepted that gibbons, the great apes and humans together form the monophyletic group Hominoidea (Groves, 1989). It has also been widely accepted in recent years that the gibbons constitute the sister group to the great apes and humans (Fig. 1) and show the most primitive characteristics within the Hominoidea (Fleagle, 1984). This view is supported by results from comparative studies of a wide array of morphological (Biegert, 1973; Remane, 1921; Sawalischin, 1911; Schultz, 1933, 1973; Wislocki, 1929, 1932), physiological (Hellekant et al., 1990), cytogenetic (Wienberg & Stanyon, 1987) and molecular data (Darga et al., 1973, 1984; Dene et al., 1976; Doolittle et al., 1971; Felsenstein, 1987; Goldman et al., 1987; Sarich & Cronin, 1976; Sibley & Ahlquist, 1984, 1987).

Figure 1. Systematic position of the gibbons within the primate order.
There is considerably less agreement on the phylogenetic relationships among gibbon species; some views are shown in Fig. 2. Several authors suggest that among modern gibbons, the siamang (S. syndactylus) was the first species to split off from the main stem (Bruce & Ayala, 1979; Creel & Preuschoft, 1976, 1984). Others disagree and see the crested gibbons (concolor group) in that position (Groves, 1972; Haimoff, 1983; Haimoff et al., 1982, 1984), and according to a third view the siamang and the crested gibbons share a common ancestor not shared by other gibbons (Shafer, 1986; van Tuinen & Ledbetter, 1983, 1989). Apparently, the "relationships of the main divisions are very even, and any dichotomy is hard to elucidate" (Groves, 1989).
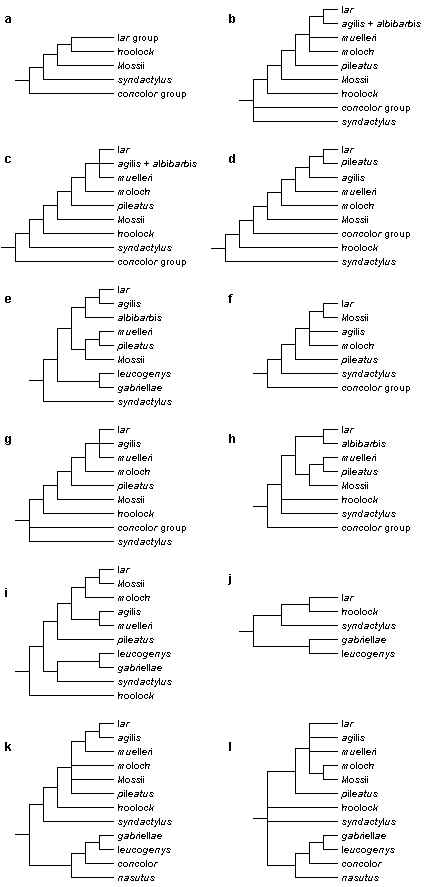
Figure 2. A comparison of published representations of the phylogenetic relationships among gibbon taxa. References: a. Groves (1972); b. Chivers (1977); c. Haimoff et al. (1982); d. Creel & Preuschoft (1984); e. Garza & Woodruff (1992); f. Hayashi et al. (1995); g. Purvis (1995); h. Zhang (1997); i. Zehr (1999); j. Roos & Geissmann (2001); k. Roos (2003); l. Takacs et al. (2005).
There is some agreement to the extent that the genus Hylobates can be divided into four systematic groups which are summarised in Table 1. The four groups have been recognised as subgenera (i.e. Hylobates, Bunopithecus, Nomascus, and Symphalangus, respectively) for several years (e.g. Geissmann, 1995a; Marshall & Sugardjito, 1986; Prouty et al., 1983). The participants of a Workshop on Primate Taxonomy (held in Orlando, Forida, Feb. 2000) proposed to elevate these subgenera to genus rank. This recommendation is followed here. Each of the four genera is, among other characteristics, identified by a distinctive karyotype; they differ in the diploid number of chromosomes, as shown in Table 1. More recently, this classification received strong support from a comparative analysis of DNA sequences (Roos & Geissmann, 2001).
As shown by Mootnick and Groves (2005), the earlier genus name Bunopithecus is not applicable to hoolock gibbons, because the type specimen of Bunopithecus - a fossil mandibular fragment from a Mid-Pleistocene fissure deposit in Sichuan province, China - is outside the range of modern Hylobatidae in its dental characters. Therefore, the authors supplied the new generic name Hoolock for hoolock gibbons.
| Genus | Diploid number of chromosomes | Other division names | Species |
| Hylobates |
44 |
Lar group, dwarf gibbons |
H. agilis H. albibarbis H. klossii H. lar H. moloch H. muelleri H. pileatus |
| Hoolock |
38 |
Hoolocks | H. hoolock H. leuconedys |
| Nomascus |
52 |
Concolor group, crested gibbons |
N. concolor N. hainanus N. nasutus N. gabriellae N. siki N. leucogenys |
| Symphalangus |
50 |
Siamang | S. syndactylus |
Within the 44-chromosome gibbons (genus Hylobates), Kloss's gibbon (H. klossii) has for some time been considered to be the first species to have differentiated from the main stock (Chivers, 1977; Creel & Preuschoft, 1976, 1984; Groves, 1989; Haimoff, 1983; Haimoff et al., 1982, 1984). The remaining group of gibbons has been referred to as the lar group (Brockelman & Gittins, 1984; Groves, 1972, 1984; Haimoff et al., 1984; Marshall & Sugardjito, 1986; Marshall et al., 1984). According to more recent studies on gibbon vocalisations (Geissmann, 1993, 2002a, 2002b) and mitochondrial DNA sequences (Garza & Woodruff, 1992), the traditional exclusion of Kloss's gibbon from the lar group may not be justified. On the other hand, a closer affinity between Kloss's gibbon and the crested gibbons (genus Nomascus), as suggested by Berger and Tylinek (1984, p. 174), is not supported by current data.
Morphological differences within the lar group are slight (Groves, 1984), karyotypes are virtually identical (Stanyon et al., 1987) and phylogenetic relationships highly speculative (Creel & Preuschoft, 1984); as a result, the lar group has been considered as a single species (i.e. H. lar) in at least one study (Creel & Preuschoft, 1984), in contrast to other recent studies which recognise 4 species (Groves, 1984), 5 species (Chivers, 1977; Chivers & Gittins, 1978; Geissmann, 1993, 1995a, 2002b; Haimoff, 1983; Haimoff et al., 1982, 1984; Marshall & Sugardjito, 1986; Marshall et al., 1984), or 6 species (Groves, 2001).
In order to discuss the phylogenetic relationships within any group of animals, it is necessary to define clearly the various taxa under comparison at the outset. Therefore, the purpose of this chapter is to review briefly the current status of gibbon classification at the species level. The classification adopted here will serve as a provisional working base for the chapters to follow.
During the last 30 years, several reviews of gibbon taxonomy have been published (Chivers, 1977; Chivers & Gittins, 1978; Groves, 1972, 1984, 1993; Marshall & Sugardjito, 1986; Napier & Napier, 1967). New evidence on gibbon systematics became available in such a steady stream that each review was in need of revision only a few years after its publication - and this will without doubt happen to the present publication.
Although still frequently cited, the gibbon taxonomy used by Napier and Napier (1967) has become outdated today because of a considerable amount of new information published after the release of this important textbook. Groves' monograph (1972) not only contains a useful review of the literature on gibbon taxonomy published before 1970, but to this day also remains the most impressive compilation and review of data relating to the topic, including the most comprehensive survey of museum specimens. Chivers (1977), Chivers and Gittins (1978) and Groves (1984, 1993) presented modifications and additions to the taxonomy proposed by Groves (1972). These changes mainly resulted from the increasing knowledge gained from various field studies.
Marshall and Sugardjito (1986) combined data from their own studies on both wild gibbons and museum specimens. Their first-hand knowledge of song- and fur-characteristics of many gibbon populations, together with detailed distribution maps, colour illustrations of the subspecies within the lar group, and a review of the recent literature, makes this probably the single most recommendable introduction to gibbon classification at this time. With only few modifications, this paper will be used here as the standard reference for the taxonomy of the lesser apes.
The major modification concerns the crested gibbons (concolor group): Whereas Marshall and Sugardjito (1986) recognised only one species (namely N. concolor), four species are recognised here. Recognition of the light-cheeked gibbon (N. leucogenys) as a separate species from the black crested gibbon (N. concolor) was proposed mainly because of anatomical differences between the two taxa - especially in the size of the penis bone (baculum) (Dao Van Tien, 1983; Ma & Wang, 1986). In addition, evidence from museum specimens appeared to suggest that areas of sympatry between the forms exist both in China and in Vietnam (Dao Van Tien, 1983; Ma & Wang, 1986), although my own examination of the same museum material did not support this conclusion (Geissmann, unpublished data).
A suggested species-level differentiation between N. leucogenys and N. gabriellae was also based on differences in the penis bone (Groves, 1993; Groves & Wang, 1990); however, only one such bone has been studied of N. gabriellae. Own studies on large samples demonstrate that all three forms (concolor, leucogenys and gabriellae) differ markedly in their song (Geissmann, 1993, 1995a; Geissmann et al., 2000).
A further form, siki, whose distribution area is situated between that of N. gabriellae and N. leucogenys, has previously been identified as a subspecies N. gabriellae by Groves (Groves, 1993; Groves & Wang, 1990), based on a penis bone at the Muséum National d'Histoire Naturelle in Paris. Unfortunately, this particular bone is not suitable to determine the affinities of siki, because it is (1) incomplete and (2) not of siki but of N. leucogenys (Geissmann, unpublished data). On the other hand, the song of zoo speciemns of siki, although having distinct characteristics, resembles that of N. leucogenys more than that of any other form of crested gibbon including N. gabriellae (Geissmann, unpublished data). Likewise, mitochondrial DNA sequences suggest that siki is more closely related to leucogenys than to gabriellae (Garza & Woodruff, 1992, 1994). As additional evidence for a close relationship between leucogenys and siki, it should also been noted that the females of both forms are so similar in fur colouration that no distinctive features are known, at present, whereas both differ from females of N. gabriellae (Geissmann, 1995a; Geissmann et al., 2000). Here, siki is recognised as a full species, following Groves (2001).
Ma and Wang (1986) described the subspecies N. concolor furvogaster from western Yunnan province (China). I consider recognition of this subspecies highly questionable. Own studies using all museum specimens of this form demonstrated that its distinguishing characteristics are based on the description of subadult females which have not attained their adult colouration. Adult females of "furvogaster" do not exhibit these characteristics but resemble females of N. c. concolor and females from central Yunnan which have been described as N. c. jingdongensis (Ma & Wang, 1986). Whether or not the latter form deserves separation from N. c. concolor is also debatable.
Own studies demonstrate that vocalisations of black crested gibbons from Cao Bang province in northeastern Vietnam and adjacent Guangxi province (China) resemble songs of N. hainanus from the island of Hainan, but both differ radically from songs of all other crested gibbons. These eastern black crested gibbons were provisionally referred to as N. sp. cf. nasutus (Geissmann, 1997, 2002b, Geissmann et al., 2000, and unpublished data; see also Geissmann, 1989). As it has become clear more recently that the Hainan and the mainland populations strongly differ in fur colouration (Geissmann, unpublished data), both populations should be recognized as full species. These are N. nasutus from the mainland and N. hainanus from Hainan.
Within the lar group, there is some controversy about the phylogenetic affinities of the Bornean race albibarbis (Groves, 1984): Whereas vocal characteristics of this gibbon are virtually identical to those of H. agilis, its fur colouration shows some similarities to H. muelleri muelleri, which also occurs in Borneo. Both forms share a common border of distribution along the Barito River in Southwest Borneo, and both hybridise at the headwaters of the Barito River (Brockelman & Gittins, 1984; Marshall & Sugardjito, 1986; Marshall et al., 1984; Mather, 1992). As a result, the options for the systematic treatment of albibarbis include, among others, making it a subspecies of either H. agilis or H. muelleri, separating albibarbis as yet another species, or combining H. agilis, H. muelleri and albibarbis into one species (Groves, 1984). In the present study, albibarbis is recognised as a full species, following Groves (2001).
Of the 16 species recognised here, N. concolor, N. nasutus, N. hainanus, N. gabriellae H. siki and N. leucogenys constitute the concolor group (genus Nomascus, crested gibbons) already mentioned above. The lar group (44-chromosome gibbons, genus Hylobates) contains the species H. agilis, H. albibarbis, H. klossii, H. lar, H. moloch, H. muelleri, and H. pileatus.
For most gibbon taxa, several different vernacular names are in use. There are no international guidelines for the creation of such names, but the inconsistency of their use, the inaccuracy or ambiguity of their meaning can sometimes be misleading. In Figure 3 and in the Gallery pages, the most frequently used vernacular names are provided for each species. The classification of the genus Hylobates used here is summarised in Table 2.
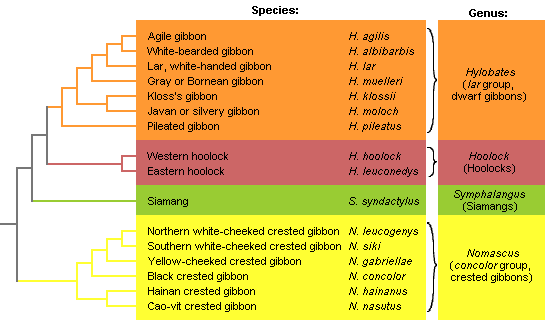
Figure 3. Preliminary phylogenetic tree of the gibbons, combining trees based
on vocal and molecular data (Geissmann, 2002b; Roos & Geissmann, 2001).
| Subspecies | Distribution | Common name | |
| Hoolock 1 | |||
| Assam, Bangladesh, Burma west of Chindwin river | Western hoolock gibbon | ||
| Burma east of Chindwin river, west Yunnan (China) | Eastern hoolock gibbon | ||
| Hylobates | |||
| agilis | West Sumatra (Indonesia) | Mountain agile gibbon | |
| ?unko 2 | Malay peninsula, east Sumatra (Indonesia) | Lowland agile gibbon | |
| Southwest Borneo between Kapuas and Barito river (Indonesia) | White-bearded gibbon | ||
| Mentawai Islands (Indonesia) | Kloss's or Mentawai gibbon | ||
| lar | Malay peninsula | Malaysian lar or white-handed gibbon | |
| carpenteri | North Thailand, east Burma, west Laos | Carpenter's lar or white-handed gibbon | |
| entelloides 4 | Central and south Thailand, southeast Burma | Central lar or white-handed gibbon | |
| vestitus | North Sumatra (Indonesia) | Sumatran lar or white-handed gibbon | |
| ?yunnanensis 5 | Southwest Yunnan (China) | Yunnan lar or white-handed gibbon | |
| moloch | West Java (Indonesia) | Western silvery or Western Javan gibbon | |
| ? pongoalsoni 6 | Central Java (Indonesia) | Eastern silvery or Central Javan gibbon | |
| muelleri | Southeast Borneo east of Barito river (Indonesia) | Southern or Muller's gray gibbon | |
| abbotti | West Borneo north of Kapuas river (Indonesia) | Abbott's gray gibbon | |
| funereus | North Borneo (Indonesia, Malaysia, Brunei) | Northern gray gibbon | |
| ? new ssp. | Central east Borneo (Indonesia) | - | |
| East Thailand, west Cambodia | Pileated or capped gibbon | ||
| Nomascus | |||
| concolor | North Vietnam, central Yunnan (China) between Black and Red rivers | Tonkin black crested gibbon | |
| ?furvogaster 7 | West Yunnan, between Salween and Mekong river | West Yunnan black crested gibbon | |
| ?jingdongensis 7 | Central Yunnan, between Mekong and Black river | Jingdong black crested gibbon | |
| ?lu 7 | Bokeo Province (northwest Laos) | Laotian black crested gibbon | |
| Hainan Island (China) | Hainan crested gibbon | ||
| Northeast Vietnam, east of Red River | Cao-vit crested gibbon | ||
| North Laos, northwest Vietnam, south Yunnan (China) | Northern white-cheeked crested gibbon | ||
| South Laos, central Vietnam | Southern white-cheeked crested gibbon | ||
| South Laos, south Vietnam, east Cambodia | Yellow-cheeked crested gibbon | ||
| Symphalangus | |||
| syndactylus | Sumatra (Indonesia) | Sumatran siamang | |
| ?continentis 8 | Malay peninsula | Malaysian siamang | |
1
The earlier genus name Bunopithecus is not applicable to hoolock gibbons,
because the type specimen of Bunopithecus (a fossil mandibular fragment from
a Mid-Pleistocene fissure deposit in Sichuan province, China) is outside the range
of modern Hylobatidae in its dental characters (Mootnick and Groves, 2005).
2
Differs from H. a. agilis in the higher frequency of darker color morphs and
the lower frequency of the lighter morphs. This distinction does not permit an identification
of individuals of unknown provenience. Additional research necessary.
3
Research necessary to distinguish features of various subspecies.
4
Elevated body weights at isthmus of Kra may indicate additional taxon (Geissmann,
unpublished data).
5
Very similar to, and possibly synonymous with, H. l. carpenteri.
6
Based only on preliminary molecular data (Andayani et al., 1998, 2001).
7
Very similar to, and possibly synonymous with, N. c. concolor.
8
May be slightly smaller than Sumatran form based on dental measurements; no body
weights of wild animals are available from the Malayan peninsula and no other distinguishing
characteristics are known.
The gibbons are distributed throughout the tropical rain forests of Southeast Asia (e.g. Chivers, 1977; Groves, 1972; Marshall & Sugardjito, 1986). A distribution map of the main systematic divisions (i.e. the genera) of the Hylobatidae is shown in Figure 4. In order to keep this map and the following maps simple, the distribution areas are mostly depicted like large continuous areas, which they probably were in the origin. Mainly as a result of habitat destruction, present distribution areas are considerably more fragmented than indicated in these figures, often consisting of isolated and (sometimes very small) patches of more or less virginal forest. Maps of the remaining areas of the tropical forests in Asia are shown in Collins et al. (1991).
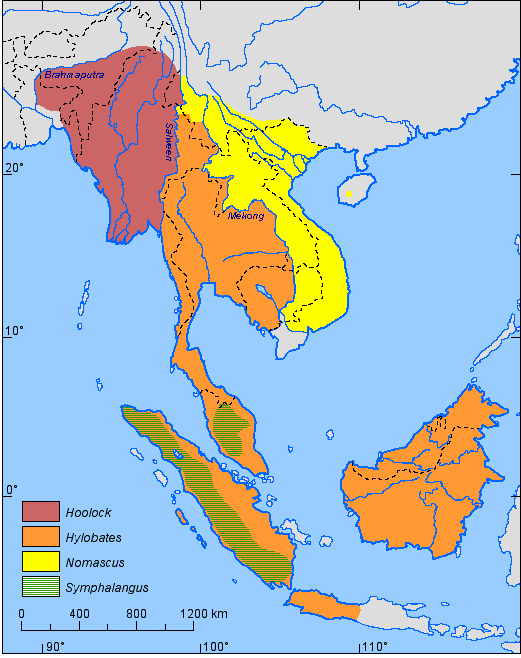
Figure 4. Distribution of the gibbon genera: Hoolock (2 species); Hylobates (7 species); Nomascus (6 species); Symphalangus (S. syndactylus). (References: Chivers, 1974; Chivers & Gittins, 1978; Fooden et al., 1987; Ma & Wang, 1986; Zhang et al., 1992).
Gibbon species are almost everywhere separated by rivers and straits. The only extensive degree of sympatry is between the siamang (genus Symphalangus) and the lar group (genus Hylobates) and was probably made possible by the strong size difference between them: Over the whole range of its distribution, the siamang occurs in sympatry with either H. agilis or H. lar. A small area of sympatry may have existed between the genus Nomascus (N. concolor) and the genus Hylobates (H. lar) in southwestern Yunnan (see Figure 4) (Ma & Wang, 1986; Zhang et al., 1992).
The species distribution for the genus Hoolock is shown in Figure 5, that for the genus Hylobates is shown in Figure 6.
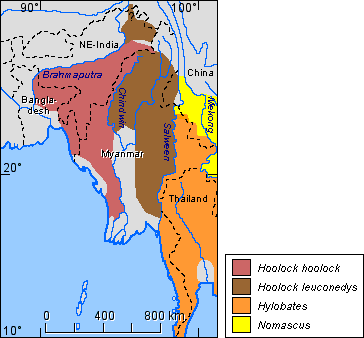
Figure 5. Distribution of the species of the genus Hoolock.
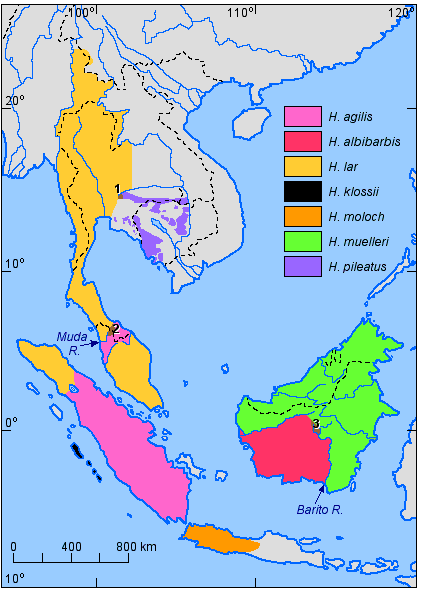
Figure 6. Distribution of the species of the genus Hylobates (References: Chivers & Gittins, 1978; Geissmann, 1991; Ma & Wang, 1986; Marshall & Sugardjito, 1986). Nos. 1-3 refer to areas of sympatry and hybridisation which are described in the text.
Within the genus Hylobates, three areas of
sympatry with some hybridisation are known. They are numbered in Figure 6 as follows:
1) H. lar and H. pileatus at the headwaters of the Takhon River in
Khao Yai National Park, about 120 km NE of Bangkok (Thailand). As late as 1925, sympatry
between these two species apparently also extended to about 80 km SE of Bangkok (Geissmann,
1991), but gibbon habitat now appears to have been destroyed in most parts of this
zone. The area of overlap in the Khao Yai National Park is about 100 km2,
where hybrids constitute about 5% of the breeding population (Brockelman & Gittins,
1984; Marshall & Sugardjito, 1986; Marshall & Brockelman, 1986).
2) H. agilis and H. lar at the headwaters of the Muda River in the
north-western part of Peninsular Malaysia. A small number of mixed groups and hybrids
have been found there on the shores of a artificial lake (Brockelman & Gittins,
1984; Gittins, 1978).
3) H. albibarbis and H. muelleri at the headwaters of the Barito River
in Kalimantan (Brockelman & Gittins, 1984; Marshall & Sugardjito, 1986).
This area is particularly interesting: A zone of at least 3,500 km2
is inhabited by an apparently stable hybrid population (Mather, 1992). No pure-species
individuals have been found in the area, suggesting that gene flow from the adjacent
pure populations into the area must be very limited.
The approximate subspecies distriution for the white-handed gibbon (Hylobates lar) is shown in Figure 7.
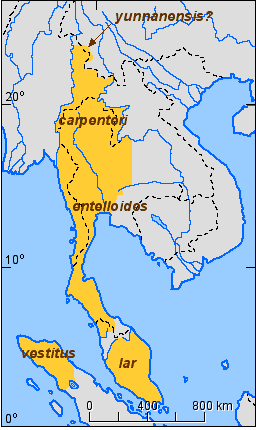
Figure 7. Approximate distribution of the subspecies of the white-handed gibbon (Hylobates lar).
The species distribution for the genus Nomascus is shown in Figure 8.
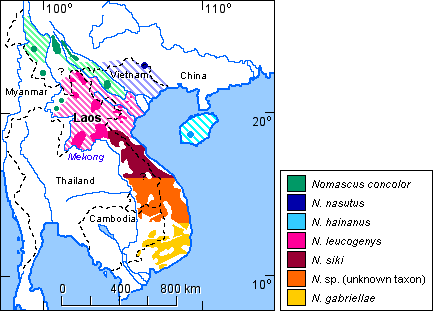
Figure 8. Distribution of the species of the genus Nomascus. (References: Fooden et al., 1987; Geissmann, 1989 and unpubl. data; Geissmann et al., 2000; Groves, 1993; Groves & Wang, 1990; Ma & Wang, 1986). Striped areas indicate assumed original distribution areas of the respective species.
Contact zones between species of the concolor group are less well known. Small areas of sympatry have been reported to occur between N. concolor and N. leucogenys in southern Yunnan (China) and northern Vietnam (Dao Van Tien, 1983; Ma & Wang, 1986). A possible wild-born hybrid between these two species has been described by Geissmann (1995b). A contact zone of unknown extent, possibly with some hybridisation, may occur between the respective distribution areas of N. gabriellae and N. siki in southern Vietnam and Laos (see Fig. 6), but not much data on that zone is available. Groves (1972) regards museum specimens from Saravane (Laos) as intergrades between gabriellae and siki. Gibbon songs from Xe Piane (southern Laos) appear to be intermediates between gabriellae and siki. Gibbon songs from the Bolovens Plateau (southern Laos, NE of Xe Piane) and from Bach Ma (central Vietnam) sound like siki, but Museum specimens from the same localities (American Museum of Natural History, New York, and Naturhistoriska Riksmuseet, Stockholm, respectively) clearly look like gabriellae. A recent review of museum skins and tape-recordings of wild gibbons suggests that a very large zone with gibbons of unknown identity exists which may be an intergrade zone between siki and gabriellae, or which may be inhabited by gibbons of an as yet undiscribed taxon (Geissmann et al., 2000).
While Chinese gibbons today are restricted to southern Yunnan and Hainan (Fooden et al., 1987; Geissmann, 1989; Groves & Wang, 1990; Ma & Wang, 1986), their former distribution range extended as far north as the Yellow River in historical times (Gao et al., 1981; van Gulik, 1967; Zhang et al., 1992), as shown in Figure 9. The identity of these gibbons is unclear. Although the more southern populations were, in all probability, members of the genus Nomascus, Pleistocene fossils (mainly confined to individual teeth) from the more northern part of this now gibbon-less area have been referred to both Nomascus and Hoolock (Groves, 1972; Gu, 1989; Marshall & Sugardjito, 1986). Several old Chinese paintings of gibbons are reproduced in Van Gulik (1967). At least the most naturalistic of these paintings strikingly resembles Hoolock hoolock. It is attributed to Yi Yuanji (ca 1000-1064 AD), who reportedly had wandered all over south Hubei and north Hunan Provinces in order to observe wild gibbons.
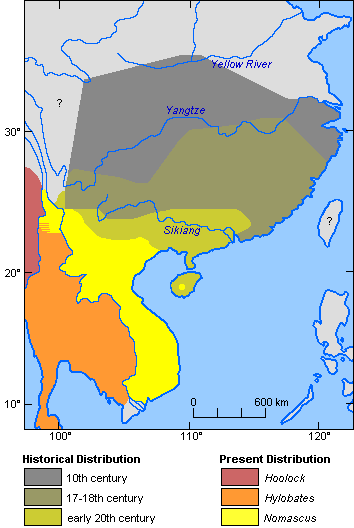
Figure 9. Historical and present distribution of gibbons in China and adjacent regions. (References: Gao et al. 1981; van Gulik, 1967; Zhang et al., 1992).